
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.

Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

A sodium phosphate is a generic variety of salts of sodium and phosphate. Phosphate also forms families or condensed anions including di-, tri-, tetra-, and polyphosphates. Most of these salts are known in both anhydrous (water-free) and hydrated forms. The hydrates are more common than the anhydrous forms.

Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula H4P2O7 or, more descriptively, [(HO)2P(O)]2O. Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs, which melt at 54.3 and 71.5 °C. The compound is a component of polyphosphoric acid, an important source of phosphoric acid. Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.

In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Calcium pyrophosphate (Ca2P2O7) is a chemical compound, an insoluble calcium salt containing the pyrophosphate anion. There are a number of forms reported: an anhydrous form, a dihydrate, Ca2P2O7·2H2O and a tetrahydrate, Ca2P2O7·4H2O. Deposition of dihydrate crystals in cartilage are responsible for the severe joint pain in cases of calcium pyrophosphate deposition disease (pseudo gout) whose symptoms are similar to those of gout. Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes, because of its insolubility and nonreactivity toward fluoride.

Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.

Curium(III) oxide is a compound composed of curium and oxygen with the chemical formula Cm2O3. It is a crystalline solid with a unit cell that contains two curium atoms and three oxygen atoms. The simplest synthesis equation involves the reaction of curium(III) metal with O2−: 2 Cm3+ + 3 O2− ---> Cm2O3. Curium trioxide can exist as five polymorphic forms. Two of the forms exist at extremely high temperatures, making it difficult for experimental studies to be done on the formation of their structures. The three other possible forms which curium sesquioxide can take are the body-centered cubic form, the monoclinic form, and the hexagonal form. Curium(III) oxide is either white or light tan in color and, while insoluble in water, is soluble in inorganic and mineral acids. Its synthesis was first recognized in 1955.
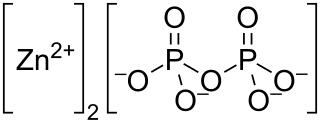
Zinc pyrophosphate (Zn2P2O7) is an ionic inorganic chemical compound composed of Zn2+ cations and pyrophosphate anions.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula NaH2PO4. It is a sodium salt of phosphoric acid. It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4). One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as monohydrate and dihydrate (NaH2PO4·H2O and NaH2PO4·2H2O respectively).

Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As a reagent in organic synthesis, it is used to convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption. The molecule is tetrahedral.

Barium metaphosphate is an inorganic substance with the molecular formula Ba(PO3)2. It is a colourless solid that is insoluble in water, though is soluble in acidic solutions through "slow dissolution". X-ray crystallography shows that this material is composed of Ba2+ cations attached to a polyphosphate ((PO3−)n) anion. A number of hydrated forms are known which are actually cyclic metaphosphates, Ba2(P4O12)·3.5H2O, Ba3(P3O9)2·6H2O.
Vanadium phosphates are inorganic compounds with the formula VOxPO4 as well related hydrates with the formula VOxPO4(H2O)n. Some of these compounds are used commercially as catalysts for oxidation reactions.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hygroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Silver hyponitrite is an ionic compound with formula Ag2N2O2 or (Ag+
)2[ON=NO]2−, containing monovalent silver cations and hyponitrite anions. It is a bright yellow solid practically insoluble in water and most organic solvents, including DMF and DMSO.

Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.

















