
Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.

In an aqueous solution, precipitation is the "sedimentation of a solid material from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.

Thiocyanates are salts containing the thiocyanate anion [SCN]−. [SCN]− is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.

Silver chloride is an inorganic chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as the mineral chlorargyrite.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
The sodium fusion test, or Lassaigne's test, is used in elemental analysis for the qualitative determination of the presence of foreign elements, namely halogens, nitrogen, and sulfur, in an organic compound. It was developed by J. L. Lassaigne.
A salt metathesis reaction is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme:
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.
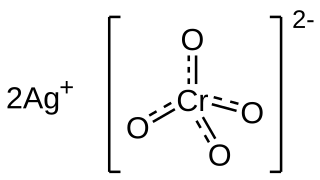
Silver chromate is an inorganic compound with formula Ag2CrO4 which appears as distinctively coloured brown-red crystals. The compound is insoluble and its precipitation is indicative of the reaction between soluble chromate and silver precursor salts (commonly potassium/sodium chromate with silver nitrate). This reaction is important for two uses in the laboratory: in analytical chemistry it constitutes the basis for the Mohr method of argentometry, whereas in neuroscience it is used in the Golgi method of staining neurons for microscopy.

Mercury(II) thiocyanate is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.

Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur:

Thiocyanogen, (SCN)2, is a pseudohalogen derived from the pseudohalide thiocyanate, [SCN]−, with behavior intermediate between dibromine and diiodine. This hexatomic compound exhibits C2 point group symmetry and has the connectivity NCS-SCN.
Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.
In analytical chemistry, argentometry is a type of titration involving the silver(I) ion. Typically, it is used to determine the amount of chloride present in a sample. The sample solution is titrated against a solution of silver nitrate of known concentration. Chloride ions react with silver(I) ions to give the insoluble silver chloride:
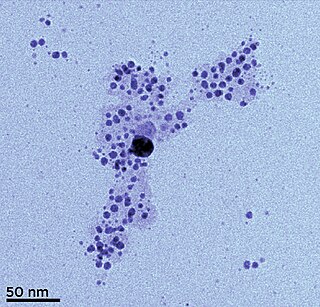
Silver nanoparticles are nanoparticles of silver of between 1 nm and 100 nm in size. While frequently described as being 'silver' some are composed of a large percentage of silver oxide due to their large ratio of surface to bulk silver atoms. Numerous shapes of nanoparticles can be constructed depending on the application at hand. Commonly used silver nanoparticles are spherical, but diamond, octagonal, and thin sheets are also common.

Silver cyanate is the cyanate salt of silver. It can be made by the reaction of potassium cyanate with silver nitrate in aqueous solution, from which it precipitates as a solid.
Throughout human history, fungi have been utilized as a source of food and harnessed to ferment and preserve foods and beverages. In the 20th century, humans have learned to harness fungi to protect human health, while industry has utilized fungi for large scale production of enzymes, acids, and biosurfactants. With the advent of modern nanotechnology in the 1980s, fungi have remained important by providing a greener alternative to chemically synthesized nanoparticle.
Lithium thiocyanate is a chemical compound with the formula LiSCN. It is an extremely hygroscopic white solid that forms the monohydrate and the dihydrate. It is the least stable of the alkali metal thiocyanates due to the large electrostatic deforming field of the lithium cation.


 [4]
[4] 












