In condensed matter physics and materials science, an amorphous solid is a solid that lacks the long-range order that is characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition. Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers.

X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the positions of the atoms in the crystal can be determined, as well as their chemical bonds, crystallographic disorder, and various other information.

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.

X-ray fluorescence (XRF) is the emission of characteristic "secondary" X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings.
In X-ray crystallography, wide-angle X-ray scattering (WAXS) or wide-angle X-ray diffraction (WAXD) is the analysis of Bragg peaks scattered to wide angles, which are caused by sub-nanometer-sized structures. It is an X-ray-diffraction method and commonly used to determine a range of information about crystalline materials. The term WAXS is commonly used in polymer sciences to differentiate it from SAXS but many scientists doing "WAXS" would describe the measurements as Bragg/X-ray/powder diffraction or crystallography.

Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of the structure of the material. The technique is similar to X-ray diffraction but due to their different scattering properties, neutrons and X-rays provide complementary information: X-Rays are suited for superficial analysis, strong x-rays from synchrotron radiation are suited for shallow depths or thin specimens, while neutrons having high penetration depth are suited for bulk samples.

A synchrotron light source is a source of electromagnetic radiation (EM) usually produced by a storage ring, for scientific and technical purposes. First observed in synchrotrons, synchrotron light is now produced by storage rings and other specialized particle accelerators, typically accelerating electrons. Once the high-energy electron beam has been generated, it is directed into auxiliary components such as bending magnets and insertion devices in storage rings and free electron lasers. These supply the strong magnetic fields perpendicular to the beam that are needed to stimulate the high energy electrons to emit photons.
In many areas of science, Bragg's law, Wulff–Bragg's condition or Laue–Bragg interference, are a special case of Laue diffraction, giving the angles for coherent scattering of waves from a large crystal lattice. It describes how the superposition of wave fronts scattered by lattice planes leads to a strict relation between the wavelength and scattering angle. This law was initially formulated for X-rays, but it also applies to all types of matter waves including neutron and electron waves if there are a large number of atoms, as well as visible light with artificial periodic microscale lattices.
Reflection high-energy electron diffraction (RHEED) is a technique used to characterize the surface of crystalline materials. RHEED systems gather information only from the surface layer of the sample, which distinguishes RHEED from other materials characterization methods that also rely on diffraction of high-energy electrons. Transmission electron microscopy, another common electron diffraction method samples mainly the bulk of the sample due to the geometry of the system, although in special cases it can provide surface information. Low-energy electron diffraction (LEED) is also surface sensitive, but LEED achieves surface sensitivity through the use of low energy electrons.
Rietveld refinement is a technique described by Hugo Rietveld for use in the characterisation of crystalline materials. The neutron and X-ray diffraction of powder samples results in a pattern characterised by reflections at certain positions. The height, width and position of these reflections can be used to determine many aspects of the material's structure.
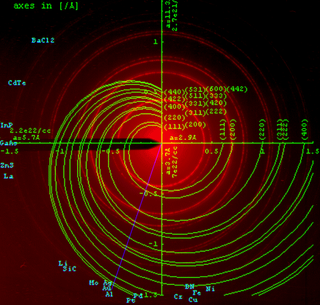
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.

Behenic acid is a carboxylic acid, the saturated fatty acid with formula C21H43COOH. In appearance, it consists of white solid although impure samples appear yellowish.
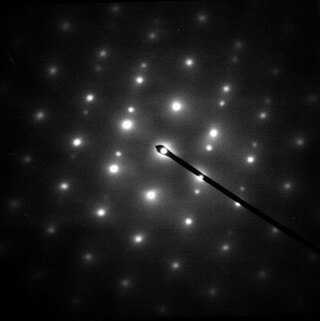
Selected area (electron) diffraction is a crystallographic experimental technique typically performed using a transmission electron microscope (TEM). It is a specific case of electron diffraction used primarily in material science and solid state physics as one of the most common experimental techniques. Especially with appropriate analytical software, SAD patterns (SADP) can be used to determine crystal orientation, measure lattice constants or examine its defects.
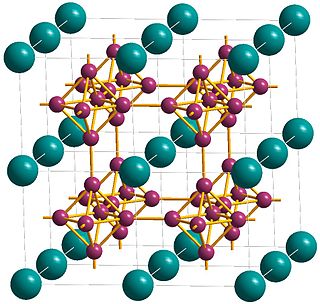
Lanthanum hexaboride (LaB6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It is extremely hard, with a Mohs hardness of 9.5. It has a low work function and one of the highest electron emissivities known, and is stable in vacuum. Stoichiometric samples are colored intense purple-violet, while boron-rich ones (above LaB6.07) are blue. Ion bombardment changes its color from purple to emerald green. LaB6 is a superconductor with a relatively low transition temperature of 0.45 K.

Kikuchi lines are patterns of electrons formed by scattering. They pair up to form bands in electron diffraction from single crystal specimens, there to serve as "roads in orientation-space" for microscopists uncertain of what they are looking at. In transmission electron microscopes, they are easily seen in diffraction from regions of the specimen thick enough for multiple scattering. Unlike diffraction spots, which blink on and off as one tilts the crystal, Kikuchi bands mark orientation space with well-defined intersections as well as paths connecting one intersection to the next.
A crystallographic database is a database specifically designed to store information about the structure of molecules and crystals. Crystals are solids having, in all three dimensions of space, a regularly repeating arrangement of atoms, ions, or molecules. They are characterized by symmetry, morphology, and directionally dependent physical properties. A crystal structure describes the arrangement of atoms, ions, or molecules in a crystal.
Le Bail analysis is a whole diffraction pattern profile fitting technique used to characterize the properties of crystalline materials, such as structure. It was invented by Armel Le Bail around 1988.
Hybrid pixel detectors are a type of ionizing radiation detector consisting of an array of diodes based on semiconductor technology and their associated electronics. The term “hybrid” stems from the fact that the two main elements from which these devices are built, the semiconductor sensor and the readout chip, are manufactured independently and later electrically coupled by means of a bump-bonding process. Ionizing particles are detected as they produce electron-hole pairs through their interaction with the sensor element, usually made of doped silicon or cadmium telluride. The readout ASIC is segmented into pixels containing the necessary electronics to amplify and measure the electrical signals induced by the incoming particles in the sensor layer.
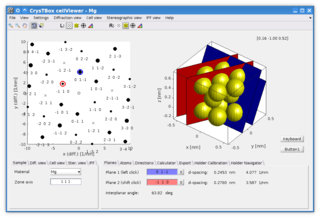
CrysTBox is a suite of computer tools designed to accelerate material research based on transmission electron microscope images via highly accurate automated analysis and interactive visualization. Relying on artificial intelligence and computer vision, CrysTBox makes routine crystallographic analyses simpler, faster and more accurate compared to human evaluators. The high level of automation together with sub-pixel precision and interactive visualization makes the quantitative crystallographic analysis accessible even for non-crystallographers allowing for an interdisciplinary research. Simultaneously, experienced material scientists can take advantage of advanced functionalities for comprehensive analyses.
X-ray diffraction computed tomography is an experimental technique that combines X-ray diffraction with the computed tomography data acquisition approach. X-ray diffraction (XRD) computed tomography (CT) was first introduced in 1987 by Harding et al. using a laboratory diffractometer and a monochromatic X-ray pencil beam. The first implementation of the technique at synchrotron facilities was performed in 1998 by Kleuker et al.










