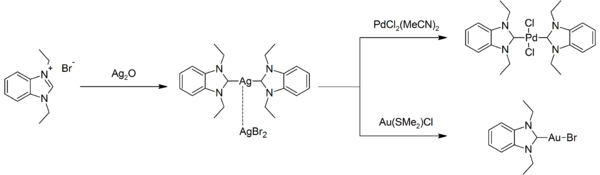The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.

The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens (1900–2000). The usage of aprotic solvents gives predominantly Z-alkenes, while protic solvent gives a mixture of E- and Z-alkenes. As an alkene-generating transformation, the Bamford–Stevens reaction has broad utility in synthetic methodology and complex molecule synthesis.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Silver is a relatively unreactive metal, although it can form several compounds. The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass. Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related BArF−
20, [(C6F5)4B]−.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.

Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene. The more general reaction, hydroalkenylation, is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene:
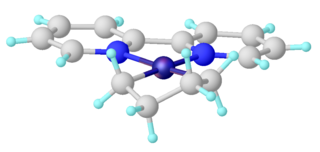
In organometallic chemistry, metallacyclopentanes are compounds with the formula LnM(CH2)4 (Ln = ligands, and M = metal). They are a type of metallacycle. Metallacyclopentanes are intermediates in some metal-catalysed reactions in homogeneous catalysis.

Phenylsilver is an organosilver compound with the chemical formula C6H5Ag. It is a white solid. The structure is assumed to be polymeric. Mesitylsilver is a tetramer.