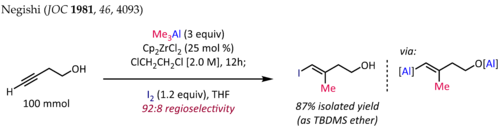Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.
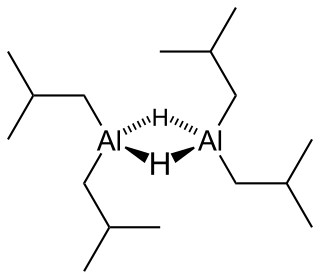
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon. This chemical reaction is useful in the organic synthesis of organic compounds.

Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.
A carbometallation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometallation.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.

Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl.

Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.
Reactions of alkenyl- and alkynylaluminium compounds involve the transfer of a nucleophilic alkenyl or alkynyl group attached to aluminium to an electrophilic atom. Stereospecific hydroalumination, carboalumination, and terminal alkyne metalation are useful methods for generation of the necessary alkenyl- and alkynylalanes.

Ethylaluminium sesquichloride, also called EASC, is an industrially important organoaluminium compound used primarily as a precursor to triethylaluminium and as a catalyst component in Ziegler–Natta type systems for olefin and diene polymerizations. Other applications include use in alkylation reactions and as a catalyst component in linear oligomerization and cyclization of unsaturated hydrocarbons. EASC is a colourless liquid, spontaneously combustible in air and reacts violently when in contact with water and many other compounds.

Triisobutylaluminium (TiBA) is an organoaluminium compound with the formula Al(CH2CH(CH3)2)3. This colorless pyrophoric liquid is mainly used to make linear primary alcohols and α-olefins.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).