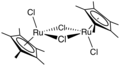
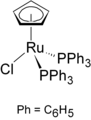Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest [1] and organoruthenium compounds have been considered for cancer therapy. [2] The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Contents
- Ligands
- Phosphine ligands
- N-Heterocyclic carbene ligands
- Cyclopentadienyl ligands
- Arene and alkene ligands
- Carbonyls
- References
In its organometallic compounds, ruthenium is known to adopt oxidation states from −2 ([Ru(CO)4]2−) to +6 ([RuN(Me)4]−). Most common are those in the +2 oxidation state, as illustrated below.