An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
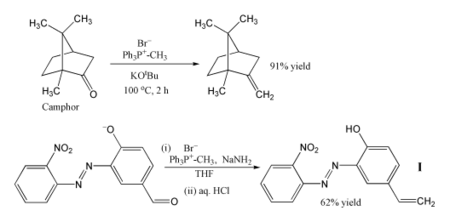
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.

The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring trans substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins.

A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. Phosphoranes have the general formula PR5.
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes to produce predominantly E-alkenes.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.
Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula R−N=P(−NR2)3. These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases. Another class of compounds called phosphazenes are represented with the formula (−N=P 2−)n, where X = halogen, alkoxy group, amide and other organyl groups. One example is hexachlorocyclotriphosphazene (−N=P 2−)3. Bis(triphenylphosphine)iminium chloride [Ph3P=N=PPh3]+Cl−is also referred to as a phosphazene, where Ph = phenyl group. This article focuses on those phosphazenes with the formula R−N=P(−NR2)3.
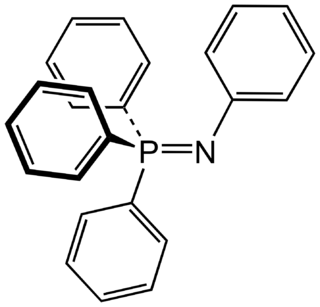
Iminophosphorane is a kind of organophosphorus compound with the formula R3PNR'. Like the corresponding phosphine oxides and Wittig reagents, iminophosphoranes are ylides. Their bonding is described by two resonance structures.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.

Organosilver chemistry is the study of organometallic compounds containing a carbon to silver chemical bond. The theme is less developed than organocopper chemistry.

Phosphinidenes are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties. In the last few decades, several strategies have been employed to stabilize phosphinidenes, and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.
Montréalone is a mesoionic heterocyclic chemical compound. It is named for the city of Montréal, Canada, which is the location of McGill University, where it was first discovered.
Hexaphenylcarbodiphosphorane is the organophosphorus compound with the formula C(PPh3)2 (where Ph = C6H5). It is a yellow, moisture-sensitive solid. The compound is classified as an ylide and as such carries significant negative charge on carbon. It is isoelectronic with bis(triphenylphosphine)iminium. The P-C-P angle is 131°. The compound has attracted attention as an unusual ligand in organometallic chemistry.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.

Methyltriphenylphosphonium bromide is the organophosphorus compound with the formula [(C6H5)3PCH3]Br. It is the bromide salt of a phosphonium cation. It is a white salt that is soluble in polar organic solvents.
In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:
In organic chemistry, methylenation is a chemical reaction that inserts a methylene group into a chemical compound:
1-Phosphaallenes is are allenes in which the first carbon atom is replaced by phosphorus, resulting in the structure: -P=C=C<.