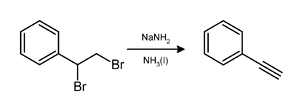
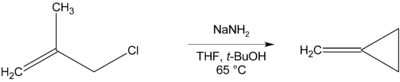
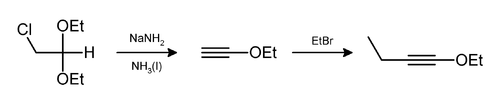Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgams are often used in reactions as strong reducing agents with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

Trimethyl borate is the organoboron compound with the formula B(OCH3)3 and a metal alkoxide. It is a colourless liquid that burns with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It is a weak Lewis acid (AN = 23, Gutmann-Beckett method).
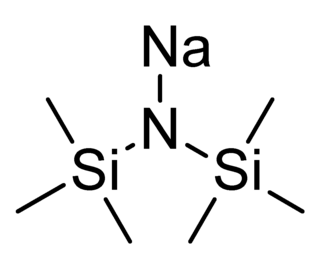
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur:

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
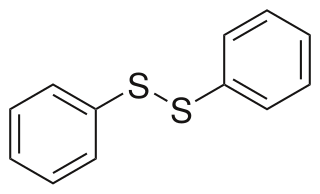
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiophenol is responsible for the disagreeable odour associated with this compound.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.

Sodium methylsulfinylmethylide is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.

Phenyl isothiocyanate (PITC) is a reagent used in reversed phase HPLC. PITC is less sensitive than o-phthaldehyde (OPA) and cannot be fully automated. PITC can be used for analysing secondary amines, unlike OPA. It is also known as Edman's reagent and is used in Edman degradation.

Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. It is a white water-soluble salt. THPC has applications as a precursor to fire-retardant materials, as well as a microbiocide in commercial and industrial water systems.

Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
In organosulfur chemistry, sulfinamide is a functional group with the structure R−S(=O)−NR2. This functionality is composed of a sulfur-carbon and sulfur-nitrogen single bonds, as well as a sulfur-oxygen double bond, resulting in a tetravalent sulfur centre. As a non-bonding electron pair is also present on the sulfur, these compounds are also chiral. They are sometimes referred to as S-chiral sulfinamides. Sulfinamides are amides of sulfinic acid.

(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl, commonly known as TEMPO, is a chemical compound with the formula (CH2)3(CMe2)2NO. This heterocyclic compound is a red-orange, sublimable solid. As a stable aminoxyl radical, it has applications in chemistry and biochemistry. TEMPO is used as a radical marker, as a structural probe for biological systems in conjunction with electron spin resonance spectroscopy, as a reagent in organic synthesis, and as a mediator in controlled radical polymerization.

Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amido complexes of the parent amido ligand NH2− are rare compared to complexes with diorganylamido ligand, such as dimethylamido. Amide ligands have two electron pairs available for bonding.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).

Azanide is the IUPAC-sanctioned name for the anion NH−2. The term is obscure; derivatives of NH−2 are almost invariably referred to as amides, despite the fact that amide also refers to the organic functional group –C(=O)−NR2. The anion NH−2 is the conjugate base of ammonia, so it is formed by the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°.