| |||
| |||
| |||
| Names | |||
|---|---|---|---|
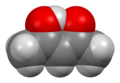
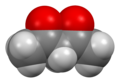
| IUPAC names (3Z)-4-Hydroxy-3-penten-2-one (enol form) Pentane-2,4-dione (keto form) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| 741937 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.214 | ||
| EC Number |
| ||
| 2537 | |||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2310 | ||
CompTox Dashboard (EPA) | |||
| |||
| Properties | |||
| C5H8O2 | |||
| Molar mass | 100.117 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.975 g/mL [1] | ||
| Melting point | −23 °C (−9 °F; 250 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) | ||
| 16 g/(100 mL) | |||
| −54.88·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
    | |||
| Danger | |||
| H226, H302, H311, H320, H331, H335, H341, H370, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P308+P313, P311, P312, P321, P322, P330, P337+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 34 °C (93 °F; 307 K) | ||
| 340 °C (644 °F; 613 K) | |||
| Explosive limits | 2.4–11.6% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Acetylacetone is an organic compound with the chemical formula CH3−C(=O)−CH2−C(=O)−CH3. It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3−C(=O)−CH=C(−OH)−CH3. The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. [2] Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds.