| |
| Names | |
|---|---|
| IUPAC name Dicarbonyl(acetylacetonato)rhodium(I) | |
| Systematic IUPAC name Dicarbonyl[(Z)-4-oxopent-2-en-2-olato-κ2O,O′]rhodium(I) | |
| Other names Rhodium acetylacetonate dicarbonyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.392 |
PubChem CID | |
| |
| |
| Properties | |
| C7H7O4Rh | |
| Molar mass | 258.034 g·mol−1 |
| Appearance | green solid |
| Density | 1.95 g/cm3 |
| Melting point | 155 °C (311 °F; 428 K) |
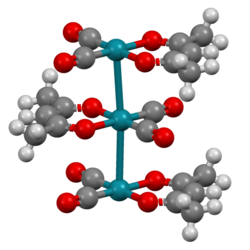
| Structure [1] | |
| triclinic | |
| P1 | |
a = 6.5189 Å, b = 7.7614 Å, c = 9.205 Å α = 106.04°, β = 91.15°, γ = 100.21° at 20°C | |
Lattice volume (V) | 439.3 Å3 |
Formula units (Z) | 2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dicarbonyl(acetylacetonato)rhodium(I) is an organorhodium compound with the formula Rh(O2C5H7)(CO)2. The compound consists of two CO ligands and an acetylacetonate. It is a dark green solid that dissolves in acetone and benzene, giving yellow solutions. The compound is used as a precursor to homogeneous catalysts. [2]
It is prepared by treating rhodium carbonyl chloride with sodium acetylacetonate in the presence of base: [3]
- [(CO)2RhCl]2 + 2 NaO2C5H7 → 2 Rh(O2C5H7)(CO)2 + 2 NaCl
The complex adopts square planar molecular geometry. The molecules stack with Rh---Rh distances of about 326 pm. As such, it is representative of a linear chain compound. [1]