Related Research Articles
Acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as: a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.

The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. The reaction was discovered by Ihsan Ullah Khand (1935-1980), who was working as a postdoctoral associate with Peter Ludwig Pauson (1925-2013) at the University of Strathclyde in Glasgow. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient and catalytic.
N,N-Diisopropylethylamine, or Hünig's base, is an organic compound and an amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a base. It is commonly abbreviated as DIPEA,DIEA, or i-Pr2NEt.
Angewandte Chemie is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society. Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. In 2018 its impact factor was 12.257, and it was ranked 17th out of 172 in the subject category "chemistry, multidisciplinary."

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.

Eschenmoser's salt, dimethyl(methylidene)ammonium iodide, is a strong dimethylaminomethylating agent, used to prepare derivatives of the type RCH2N(CH3)2. Enolates, enolsilylethers, and even more acidic ketones undergo efficient dimethylaminomethylation. Once prepared, such tertiary amines can be further methylated and then subjected to base-induced elimination to afford methylidenated ketones. The salt was first prepared by the group of Albert Eschenmoser after whom the reagent is named.

Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.

The acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of linearly fused benzene rings. The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering. Pentacene has been incorporated into organic field-effect transistors, reaching charge carrier mobilities as high as 5 cm2/Vs.
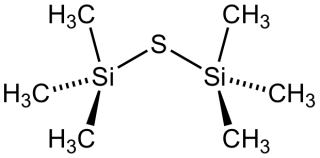
Bis(trimethylsilyl) sulfide is the chemical compound with the formula ((CH3)3Si)2S. Often abbreviated (tms)2S, this colourless, vile-smelling liquid is a useful aprotic source of “S2−“ in chemical synthesis.
Schlosser's base describes various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term LICKOR superbase. The superbasic nature of the reagent is a consequence of the in situ formation of the corresponding organopotassium compound, as well as changes to the aggregation state of the alkyllithium species.
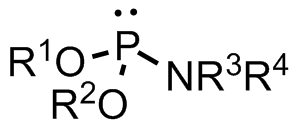
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids e.c., triethylammonium chloride or 1H-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety.
The Schöllkopf method or Schöllkopf Bis-Lactim Amino Acid Synthesis is a method in organic chemistry for the asymmetric synthesis of chiral amino acids. The method was established in 1981 by Ulrich Schöllkopf. In it glycine is a substrate, valine a chiral auxiliary and the reaction taking place an alkylation.
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.

MoOPH, also known as oxodiperoxymolybdenum(pyridine)-(hexamethylphosphoric triamide), is a reagent used in organic synthesis. It contains a molybdenum(VI) center with multiple oxygen ligands, coordinated with pyridine and HMPA ligands. It is an electrophilic source of oxygen that reacts with enolates and related structures, and thus can be used for alpha-hydroxylation of carbonyl-containing compounds. Other reagents used for alpha-hydroxylation via enol or enolate structures include Davis oxaziridine, oxygen, and various peroxyacids. This reagent was first utilized by Edwin Vedejs as an efficient alpha-hydroxylating agent in 1974 and an effective preparative procedure was later published in 1978.

Cyanogen azide, N3CN or CN4, is an azide compound of carbon and nitrogen which is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F.D. Marsh at DuPont in the early 1960s.
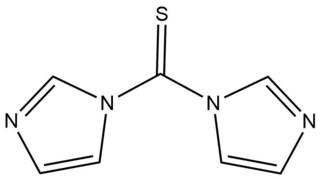
1,1'-Thiocarbonyldiimidazole (TCDI) is a thiourea containing two imidazole rings. It is the sulfur analog of the peptide coupling reagent carbonyldiimidazole (CDI).
Carbonyl olefin metathesis is a type of metathesis reaction that entails, formally, the redistribution of fragments of an alkene and a carbonyl by the scission and regeneration of carbon-carbon and carbon-oxygen double bonds respectively. It is a powerful method in organic synthesis using simple carbonyls and olefins and converting them into less accessible products with higher structural complexity.
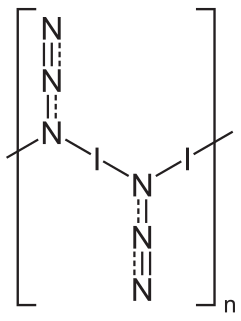
Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.
References
- ↑ Schmalz, Hans-Günther (1996). "Book Review: Encyclopedia of Reagents for Organic Synthesis. Vols. 1–8. Supervising editor: L. A. Paquette". Angewandte Chemie International Edition in English. 35 (15): 1736–1737. doi:10.1002/anie.199617361.
- ↑ Snyder, Scott A. (2017). "Essential Reagents for Organic Synthesis. Edited by Philip L. Fuchs, André B. Charette, Tomislav Rovis, Jeffrey W. Bode". Angewandte Chemie International Edition. 56 (28): 8045. doi:10.1002/anie.201703968.