 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Tris(acetylacetonato)iron(III) | |
| Systematic IUPAC name Tris[(Z)-4-oxopent-2-en-2-olato-κ2O,O′]iron(III) | |
| Other names Iron(III) acetylacetonate, Iron(III) tris(2,4-pentanedionato), Fe(acac)3 | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.034.398 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Fe(C5H7O2)3 | |
| Molar mass | 353.17 g/mol |
| Appearance | Red Solid |
| Density | 1.348 g/cm3 |
| Melting point | 180 to 181 °C (356 to 358 °F; 453 to 454 K) |
| Boiling point | decomposes |
| 2 g/L | |
| Hazards | |
| GHS labelling: [1] | |
  | |
| Danger | |
| H302+H312+H332, H318 | |
| P261, P280, P301+P312, P302+P352+P312, P304+P340+P312, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |

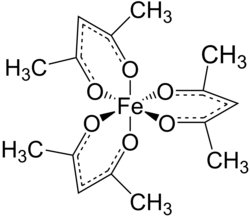
Tris(acetylacetonato)iron(III), often abbreviated Fe(acac)3, is a ferric coordination complex featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a red air-stable solid that dissolves in nonpolar organic solvents.

