Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

In chemistry, iron (III) refers to the element iron in its +3 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agent. It is used as a water cleaner and as an etchant for metals.

Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide. Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O.
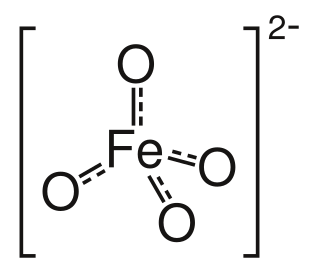
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula MIMIII(SO4)2·12H2O) and Tutton's salts (with the general formula (MI)2MII(SO4)2·6H2O). Other examples include potassium sodium tartrate, ammonium iron(II) sulfate (Mohr's salt), potassium uranyl sulfate (used to discover radioactivity) and bromlite BaCa(CO3)2. The fluorocarbonates contain fluoride and carbonate anions. Many coordination complexes form double salts.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO
3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+
and carbonate anions CO2−
3.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe2+.

Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water. Thyy

Ferric ammonium oxalate is the ammonium salt of the anionic trisoxalato coordination complex of iron(III). It is a precursor to iron oxides, diverse coordination polymers, and Prussian Blue. The latter behavior is relevant to the manufacture of blueprint paper. Ferric ammonium oxalate has also been used in the synthesis of superconducting salts with bis(ethylene)dithiotetrathiafulvalene (BEDT-TTF), see Organic superconductor.

Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe2(C2O4)3(H2O)x but could also refer to salts of Fe(C2O4)3]3-. Fe2(C2O4)3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. This article emphasizes the coordination polymers.
Potassium ferrooxalate, also known as potassium bisoxalatoferrate(II), is a salt with the formula K2Fe(C2O4)2(H2O)x. The anion is a transition metal oxalate complex, consisting of an atom of iron in the +2 oxidation state bound to oxalate (C
2O2−
4) ligands and water.

Ferrioxalate or trisoxalatoferrate(III) is a trivalent anion with formula [Fe(C2O4)3]3−. It is a transition metal complex consisting of an iron atom in the +3 oxidation state and three bidentate oxalate ions C2O2−4 anions acting as ligands.

Transition metal oxalate complexes are coordination complexes with oxalate (C2O42−) ligands. Some are useful commercially, but the topic has attracted regular scholarly scrutiny. Oxalate (C2O42-) is a kind of dicarboxylate ligand. As a small, symmetrical dinegative ion, oxalate commonly forms five-membered MO2C2 chelate rings. Mixed ligand complexes are known, e.g., [Co(C2O4)(NH3)4]κ+.
















