Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

Sodium cyanide is a poisonous compound with the formula NaCN. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Cyanate is an anion with the structural formula [O−C≡N]−, usually written OCN−. It also refers to any salt containing it, such as ammonium cyanate.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.
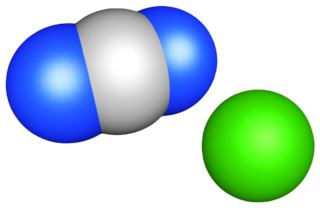
Calcium cyanamide, also known as Calcium carbondiamide, Calcium cyan-2°-amide or Calcium cyanonitride is the inorganic compound with the formula CaCN2. It is the calcium salt of the cyanamide (CN2−
2) anion. This chemical is used as fertilizer and is commercially known as nitrolime. It was first synthesized in 1898 by Adolph Frank and Nikodem Caro (Frank–Caro process).
Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.
The Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was described in 1828 by Friedrich Wöhler. It is often cited as the starting point of modern organic chemistry. Although the Wöhler reaction concerns the conversion of ammonium cyanate, this salt appears only as an (unstable) intermediate. Wöhler demonstrated the reaction in his original publication with different sets of reactants: a combination of cyanic acid and ammonia, a combination of silver cyanate and ammonium chloride, a combination of lead cyanate and ammonia and finally from a combination of mercury cyanate and cyanatic ammonia.

Isocyanic acid is a chemical compound with the structural formula HNCO, which is often written as H−N=C=O. It is a colourless, volatile and poisonous substance, with a boiling point of 23.5 °C. It is the predominant tautomer and an isomer of cyanic acid (aka. cyanol).
Sodium perborate is chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2[B2O4(OH)4]2−. Its name is sometimes abbreviated as PBS.

Cadmium oxide is an inorganic compound with the formula CdO. It is one of the main precursors to other cadmium compounds. It crystallizes in a cubic rocksalt lattice like sodium chloride, with octahedral cation and anion centers. It occurs naturally as the rare mineral monteponite. Cadmium oxide can be found as a colorless amorphous powder or as brown or red crystals. Cadmium oxide is an n-type semiconductor with a band gap of 2.18 eV at room temperature.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.
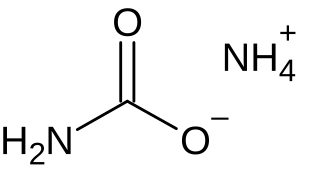
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.

Sodium germanate is an inorganic compound with the chemical formula Na2GeO3. It exists as a colorless solid. Sodium germanate is primarily used for the synthesis of other germanium compounds.

Sodium hyponitrite is a solid ionic compound with formula Na
2N
2O
2 or (Na+
)2[ON=NO]2−.
![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)
Ammoniumcyanate is an inorganic compound with the formula [NH4]+[OCN]−. It is a colorless, solid salt.

In chemistry, ureas are a class of organic compounds with the formula (R2N)2CO where R = H, alkyl, aryl, etc. Thus, in addition to describing the specific chemical compound urea ((H2N)2CO), urea is the name of a functional group that is found in many compounds and materials of both practical and theoretical interest. Generally ureas are colorless crystalline solids, which, owing to the presence of fewer hydrogen bonds, exhibit melting points lower than that of urea itself.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anhydrous compound is a green powdery solid at room temperature.




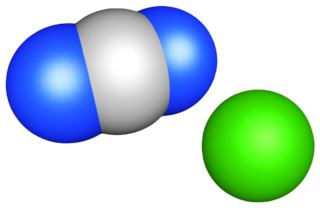






![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)
