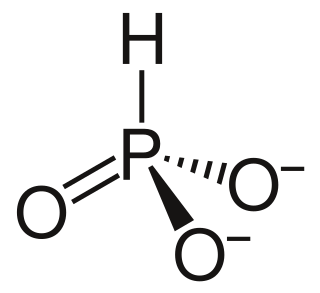
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.

Xanthate usually refers to a salt of xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+ ,. Xanthate also refers to the anion [R−O−CS2]−. Xanthate also may refer to an ester of xanthic acid. The formula of xanthic acid is R−O−C(=S)−S−H, while the formula of the esters of xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός xanthos, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.

Cacodylic acid is an organoarsenic compound with the formula (CH3)2AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water.
In chemistry, an arsenite is a chemical compound containing an arsenic oxyanion where arsenic has oxidation state +3. Note that in fields that commonly deal with groundwater chemistry, arsenite is used generically to identify soluble AsIII anions. IUPAC have recommended that arsenite compounds are to be named as arsenate(III), for example ortho-arsenite is called trioxidoarsenate(III). Ortho-arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent.

Sodium thioantimoniate or sodium tetrathioantimonate(V) is an inorganic compound with the formula Na3SbS4. The nonahydrate of this chemical, Na3SbS4·9H2O, is known as Schlippe's salt, named after Johann Karl Friedrich von Schlippe (1799–1867). These compounds are examples of sulfosalts. They were once of interest as species generated in qualitative inorganic analysis.

Sodium arsenite usually refers to the inorganic compound with the formula NaAsO2. Also called sodium meta-arsenite, it is the sodium salt of arsenous acid. Sodium ortho-arsenite is Na3AsO3. The compounds are colourless solids.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Sodium telluride is the chemical compound with the formula Na2Te. This salt is the conjugate base of the thermally unstable acid hydrogen telluride, but it is usually prepared by reduction of tellurium with sodium. Na2Te is a challenging material to handle because it is very sensitive to air. Air oxidizes it initially to polytellurides, which have the formula Na2Tex (x > 1), and ultimately Te metal. Samples of Na2Te, which are colourless when absolutely pure, generally appear purple or dark gray due to the effects of air oxidation.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.
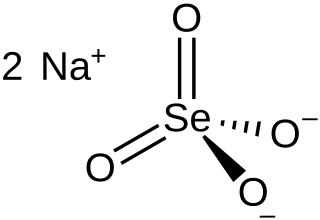
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Sodium aurothiosulfate, or sanocrysin, is the inorganic compound with the formula Na3[Au(S2O3)2]·2H2O. This salt contains an anionic coordination complex of gold(I) bound to two thiosulfate ligands. It is colorless.

Sodium aluminium sulfate is the inorganic compound with the chemical formula NaAl(SO4)2·12H2O (sometimes written Na2SO4·Al2(SO4)3·24H2O). Also known as soda alum, sodium alum, or SAS, this white solid is used in the manufacture of baking powder and as a food additive. Its official mineral name is alum-Na (IMA symbol: Aum-Na).

Sodium monothiophosphate, or sodium phosphorothioate, is an inorganic compound with the molecular formula Na3PO3S(H2O)x. All are white solids. The anhydrous material (x = 0) decomposes without melting at 120-125 °C. More common is the dodecahydrate. A nonahydrate is also known.
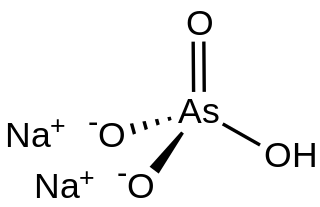
Disodium hydrogen arsenate is the inorganic compound with the formula Na2HAsO4.7H2O. The compound consists of a salt and seven molecules of water of crystallization although for simplicity the formula usually omits the water component. The other sodium arsenates are NaH2AsO4 and Na3AsO4, the latter being called sodium arsenate. Disodium hydrogen arsenate is highly toxic. The salt is the conjugate base of arsenic acid. It is a white, water-soluble solid.

Sodium dihydrogen arsenate is the inorganic compound with the formula NaH2AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). Sodium dihydrogen arsenate is a colorless solid that is highly toxic.
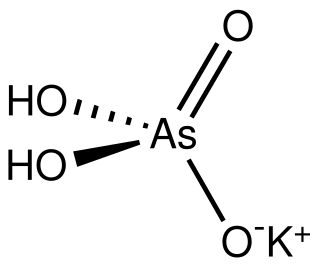
Monopotassium arsenate is the inorganic compound with the formula KH2AsO4. A white solid, this salt is used to prepared other arsenic-containing compounds, mainly pesticides. It is prepared by calcining arsenic oxide and potassium nitrate, followed by extraction with water.
Trisodium borate is a chemical compound of sodium, boron, and oxygen, with formula Na3BO3, or (Na+)3[BO3]3−. It is a sodium salt of the orthoboric acid B(OH)3.





















