Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

Borax is a salt, a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17. It is a colorless crystalline solid, that dissolves in water to make a basic solution.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in all solvents (other than molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid.
Sodium borate is a generic name for any salt of sodium with an anion consisting of boron and oxygen, and possibly hydrogen, or any hydrate thereof. It can be seen as a hydrated sodium salt of the appropriate boroxy acid, although the latter may not be a stable compound.
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules.
Sodium perborate is chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2[B2O4(OH)4]2−. Its name is sometimes abbreviated as PBS.

Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use.

Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic.

Disodium octaborate is a borate of sodium, a chemical compound of sodium, boron, and oxygen — a salt with elemental formula Na2B8O13 or (Na+)2[B8O13]2−, also written as Na2O·4B2O3. It is a colorless crystalline solid, soluble in water.
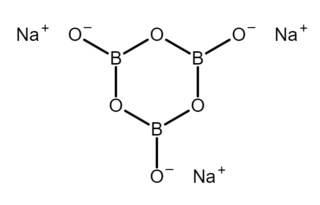
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.
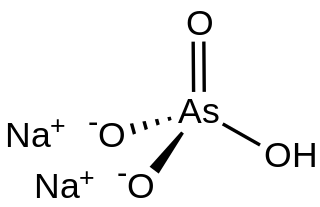
Disodium hydrogen arsenate is the inorganic compound with the formula Na2HAsO4.7H2O. The compound consists of a salt and seven molecules of water of crystallization although for simplicity the formula usually omits the water component. The other sodium arsenates are NaH2AsO4 and Na3AsO4, the latter being called sodium arsenate. Disodium hydrogen arsenate is highly toxic. The salt is the conjugate base of arsenic acid. It is a white, water-soluble solid.
Sodium pentaborate, more properly disodium decaborate, is a chemical compound of sodium, boron, and oxygen; a salt with elemental formula NaB5O8, Na2B10O16, or Na2O·5B2O3. It is a colorless crystalline solid, soluble in water.

Sodium tetrahydroxyborate is a salt of with chemical formula NaH4BO4 or Na+[B(OH)4]−. It is one of several sodium borates. At room temperature it is a colorless crystalline solid.