
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.

Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

Borax and tincar is a salt, a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17. It is a colorless crystalline solid that dissolves in water to make a basic solution.

Ulexite (NaCaB5O6(OH)6·5H2O, hydrated sodium calcium borate hydroxide), sometimes known as TV rock or television stone, is a mineral occurring in silky white rounded crystalline masses or in parallel fibers. The natural fibers of ulexite conduct light along their long axes, by internal reflection. Ulexite was named for the German chemist Georg Ludwig Ulex (1811–1883) who first discovered it.
Boron deficiency is a common deficiency of the micronutrient boron in plants. It is the most widespread micronutrient deficiency around the world and causes large losses in crop production and crop quality. Boron deficiency affects vegetative and reproductive growth of plants, resulting in inhibition of cell expansion, death of meristem, and reduced fertility.

Bentonite is an absorbent swelling clay consisting mostly of montmorillonite which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelling capacity than Ca-montmorillonite.

Gum arabic is a natural gum originally consisting of the hardened sap of two species of the Acacia tree, Senegalia senegal and Vachellia seyal. However, the term "gum arabic" does not actually indicate a particular botanical source. The gum is harvested commercially from wild trees, mostly in Sudan and throughout the Sahel, from Senegal to Somalia. The name "gum Arabic" was used in the Middle East at least as early as the 9th century. Gum arabic first found its way to Europe via Arabic ports, and so retained its name.

Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig’s law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil.

Wollastonite is a calcium inosilicate mineral (CaSiO3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or dolomite is subjected to high temperature and pressure, which sometimes occurs in the presence of silica-bearing fluids as in skarns or in contact with metamorphic rocks. Associated minerals include garnets, vesuvianite, diopside, tremolite, epidote, Plagioclase feldspar, pyroxene and calcite. It is named after the English chemist and mineralogist William Hyde Wollaston (1766–1828).
Sodium perborate is chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2[B2O4(OH)4]2−. Its name is sometimes abbreviated as PBS.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.
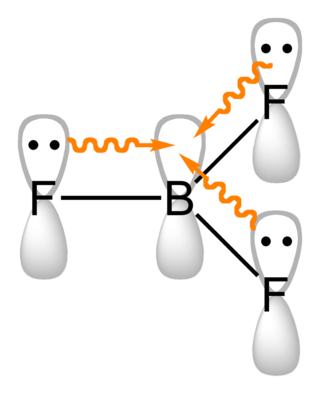
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.

Ash glazes are ceramic glazes made from the ash of various kinds of wood or straw. They have historically been important in East Asia, especially Chinese pottery, Korean pottery, and Japanese pottery. Many traditionalist East Asian potteries still use ash glazing, and it has seen a large revival in studio pottery in the West and East. Some potters like to achieve random effects by setting up the kiln so that ash created during firing falls onto the pots; this is called "natural" or "naturally occurring" ash glaze. Otherwise the ash is mixed with water, and often clay, and applied as a paste.

Ceramic glaze, or simply glaze, is a glassy coating on ceramics. It is used for decoration, to ensure the item is impermeable to liquids and to minimise the adherence of pollutants.
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium.
Fluxes are substances, usually oxides, used in glasses, glazes and ceramic bodies to lower the high melting point of the main glass forming constituents, usually silica and alumina. A ceramic flux functions by promoting partial or complete liquefaction. The most commonly used fluxing oxides in a ceramic glaze contain lead, sodium, potassium, lithium, calcium, magnesium, barium, zinc, strontium, and manganese. These are introduced to the raw glaze as compounds, for example lead as lead oxide. Boron is considered by many to be a glass former rather than a flux.

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.
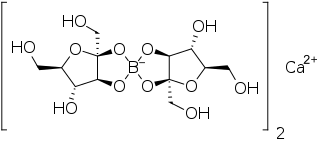
Calcium fructoborate is a salt of an organoboron compound containing boron (and fructose and calcium). Its structural formula is Ca[(C6H10O6)2B]2.













