
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource.

Calcium oxide, commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium metal.
Cement chemist notation (CCN) was developed to simplify the formulas cement chemists use on a daily basis. It is a shorthand way of writing the chemical formula of oxides of calcium, silicon, and various metals.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.
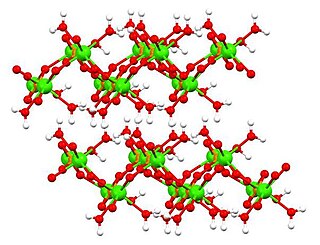
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.

Ye'elimite is the naturally occurring form of anhydrous calcium sulfoaluminate, Ca
4(AlO
2)
6SO
4. It gets its name from Har Ye'elim in Israel in the Hatrurim Basin west of the Dead Sea where it was first found in nature by Shulamit Gross, an Israeli mineralogist and geologist who studied the Hatrurim Formation.
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation).
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. It does not occur in nature, but is an important mineral phase in Portland cement.

Calcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
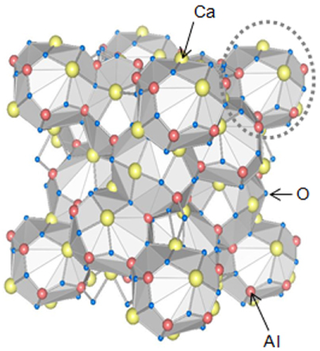
Dodecacalcium hepta-aluminate (12CaO·7Al2O3, Ca12Al14O33 or C12A7) is an inorganic solid that occurs rarely in nature as the mineral mayenite. It is an important phase in calcium aluminate cements and is an intermediate in the manufacture of Portland cement. Its composition and properties have been the subject of much debate, because of variations in composition that can arise during its high-temperature formation.

Calcium aluminate cements are cements consisting predominantly of hydraulic calcium aluminates. Alternative names are "aluminous cement", "high-alumina cement", and "Ciment fondu" in French. They are used in a number of small-scale, specialized applications.

Calcium aluminoferrite is a dark brown crystalline phase commonly found in cements. In the cement industry it is termed tetra-calcium aluminoferrite or ferrite. In cement chemist notation (CCN), it is abbreviated as C
4AF meaning 4CaO·Al
2O
3·Fe
2O
3 in the oxide notation. It also exists in nature as the rare mineral brownmillerite.
An AFm phase is an "alumina, ferric oxide, monosubstituted" phase, or aluminate ferrite monosubstituted, or Al2O3, Fe2O3 mono, in cement chemist notation (CCN). AFm phases are important hydration products in the hydration of Portland cements and hydraulic cements.
Friedel's salt is an anion exchanger mineral belonging to the family of the layered double hydroxides (LDHs). It has affinity for anions as chloride and iodide and is capable of retaining them to a certain extent in its crystallographical structure.
Krotite is a natural mineral composed of calcium, aluminium and oxygen, with the molecular formula CaAl2O4. It is the low-pressure dimorph of CaAl2O4, of which the high-pressure dimorph is named dmitryivanovite.
Dmitryivanovite is a natural mineral composed of calcium, aluminium and oxygen, with the molecular formula CaAl2O4. It was reported in 2009 in a calcium-aluminium-rich inclusion in the carbonaceous chondrite meteorite 470 (NWA470) CH3, which landed in North Africa. The mineral name was chosen to honor Dmitriy A. Ivanov (1962–1986), a geologist, mineralogist, and petrologist who died on a field expedition.
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic characteristics of the pozzolan such as the specific surface area, the chemical composition and the active phase content.
AFt Phases refer to the calcium Aluminate Ferrite trisubstituted, or calcium aluminate trisubstituted, phases present in hydrated cement paste (HCP) in concrete.








