Phosphorus is a chemical element; it has symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about 0.1%, less abundant than hydrogen but more than manganese. In minerals, phosphorus generally occurs as phosphate.

Calcium carbide, also known as calcium acetylide, is a chemical compound with the chemical formula of CaC2. Its main use industrially is in the production of acetylene and calcium cyanamide.
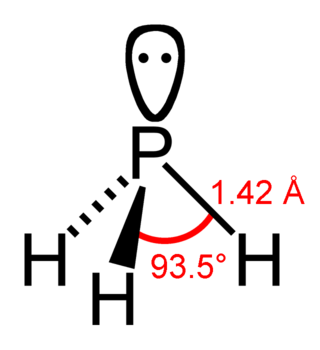
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula PH3, classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (P2H4). With traces of P2H4 present, PH3 is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure.

Rodenticides are chemicals made and sold for the purpose of killing rodents. While commonly referred to as "rat poison", rodenticides are also used to kill mice, woodchucks, chipmunks, porcupines, nutria, beavers, and voles. Despite the crucial roles that rodents play in nature, there are times when they need to be controlled.

In chemistry, a phosphide is a compound containing the P3− ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides, which are solids consisting of anionic chains or clusters of phosphorus. Phosphides are known with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, and Po. Finally, some phosphides are molecular.

Sodium phosphide is the inorganic compound with the formula Na3P. It is a black solid. It is often described as Na+ salt of the P3− anion. Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.

Aluminium phosphide is a highly toxic inorganic compound with the chemical formula AlP, used as a wide band gap semiconductor and a fumigant. This colorless solid is generally sold as a grey-green-yellow powder due to the presence of impurities arising from hydrolysis and oxidation.

Zinc phosphide (Zn3P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2).
The Holmes' Marine Life Protection Association was a United Kingdom company set up in the 19th century to produce marine signal lights and foghorns. It was founded by Nathaniel John Holmes, a telegraph engineer from Middlesex; and it passed to his son Joseph R. Holmes. The company was taken over by Albright and Wilson in 1919.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.
Iron phosphide is a chemical compound of iron and phosphorus, with a formula of FeP.< Its physical appearance is grey needles.

Calcium monophosphide is the inorganic compound with the formula CaP. It is sometimes also known as "calcium phosphide", which also describes a different compound with composition Ca3P2. Calcium monophosphide is a black solid.
Strontium phosphide is an inorganic compound of strontium and phosphorus with the chemical formula Sr
3P
2. The compound looks like black crystalline material.

Calcium cyanide is the inorganic compound with the formula Ca(CN)2. It is the calcium salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It slowly hydrolyses in solution or moist air to release hydrogen cyanide and is very toxic.
Lithium phosphide is an inorganic compound of lithium and phosphorus with the chemical formula Li3P. This dark colored compound is formally the lithium salt of phosphine, consisting of lithium cations Li+ and phosphide anions P3−. It is hazardous to handle because of its high reactivity toward air.
Lutetium phosphide is an inorganic compound of lutetium and phosphorus with the chemical formula LuP. The compound forms dark crystals, does not dissolve in water.
Europium phosphide is an inorganic compound of europium and phosphorus with the chemical formula EuP. Other phosphides are also known.
Ytterbium(III) phosphide is an inorganic compound of ytterbium and phosphorus with the chemical formula YbP. This is one of the phosphides of ytterbium.

White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is one of allotropes of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white "diphosphorus pentoxide", which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. P4 is soluble in benzene, oils, carbon disulfide, and disulfur dichloride.