An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin. While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.

Thromboembolism is a condition in which a blood clot (thrombus) breaks off from its original site and travels through the bloodstream to obstruct a blood vessel, causing tissue ischemia and organ damage. Thromboembolism can affect both the venous and arterial systems, with different clinical manifestations and management strategies.
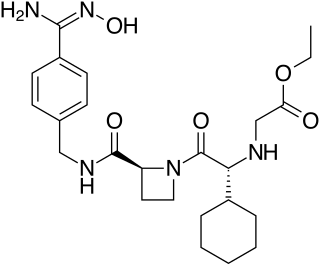
Ximelagatran is an anticoagulant that has been investigated extensively as a replacement for warfarin that would overcome the problematic dietary, drug interaction, and monitoring issues associated with warfarin therapy. In 2006, its manufacturer AstraZeneca announced that it would withdraw pending applications for marketing approval after reports of hepatotoxicity during trials, and discontinue its distribution in countries where the drug had been approved.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation. Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests. In a meta analysis of 7 different studies, there was no benefit of dabigatran over warfarin in preventing ischemic stroke; however, dabigatran were associated with a lower hazard for intracranial bleeding compared with warfarin, but also had a higher risk of gastrointestinal bleeding relative to warfarin. It is taken by mouth.
The CHADS2 score and its updated version, the CHA2DS2-VASc score, are clinical prediction rules for estimating the risk of stroke in people with non-rheumatic atrial fibrillation (AF), a common and serious heart arrhythmia associated with thromboembolic stroke. Such a score is used to determine whether or not treatment is required with anticoagulation therapy or antiplatelet therapy, since AF can cause stasis of blood in the upper heart chambers, leading to the formation of a mural thrombus that can dislodge into the blood flow, reach the brain, cut off supply to the brain, and cause a stroke.
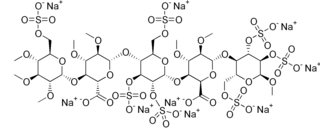
Idraparinux sodium is an anticoagulant medication in development by Sanofi-Aventis.
Direct factor Xa inhibitors (xabans) are anticoagulants, used to both treat and prevent blood clots in veins, and prevent stroke and embolism in people with atrial fibrillation (AF).

Left atrial appendage occlusion (LAAO), also referred to as left atrial appendage closure (LAAC), is a procedure used to reduce the risk of blood clots from the left atrial appendage entering the bloodstream and causing a stroke in those with non-valvular atrial fibrillation.
The management of atrial fibrillation (AF) is focused on preventing temporary circulatory instability, stroke and other ischemic events. Control of heart rate and rhythm are principally used to achieve the former, while anticoagulation may be employed to decrease the risk of stroke. Within the context of stroke, the discipline may be referred to as stroke prevention in atrial fibrillation (SPAF). In emergencies, when circulatory collapse is imminent due to uncontrolled rapid heart rate, immediate cardioversion may be indicated.

Edoxaban, sold under the brand name Lixiana among others, is an anticoagulant medication and a direct factor Xa inhibitor. It is taken by mouth.
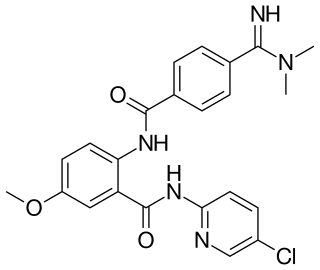
Betrixaban is an oral anticoagulant drug which acts as a direct factor Xa inhibitor. Betrixaban is FDA approved for venous thrombosis prevention in adults hospitalized for an acute illness who are at risk for thromboembolic complications. Compared to other directly acting oral anticoagulants betrixaban has relatively low renal excretion and is not metabolized by CYP3A4.

Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. Specifically, it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests or dietary restrictions. It is taken by mouth.

Darexaban (YM150) is a direct inhibitor of factor Xa created by Astellas Pharma. It is an experimental drug that acts as an anticoagulant and antithrombotic to prevent venous thromboembolism after a major orthopaedic surgery, stroke in patients with atrial fibrillation and possibly ischemic events in acute coronary syndrome. It is used in form of the maleate. The development of darexaban was discontinued in September 2011.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.
The SAMe-TT2R2 score is a clinical prediction rule to predict the quality of vitamin K antagonist anticoagulation therapy as measured by time in therapeutic INR range (TTR) (VKA e.g. warfarin). It has been suggested that it can aid in the medical decision making between VKAs and new oral anticoagulant/non-VKA oral anticoagulant (NOAC e.g. dabigatran, rivaroxaban, apixaban or edoxaban) in patients with atrial fibrillation (AF). This score can be used with patients with ≥1 additional stroke risk factors using the CHA2DS2-VASc score, where oral anticoagulation is recommended or should be considered.

Tecarfarin is a vitamin K antagonist under development for use as an anticoagulant. A Phase II/III clinical trial in 607 people, comparing it to the established vitamin K antagonist warfarin, found no difference in quality of anticoagulation or side effects between the two drugs in the overall population. Among patients taking CYP2C9 interacting drugs however, the tecarfarin patients’ TTR was 72.2% (n=92) vs 69.9% (n=87) for warfarin patients (pint=0.16); among patients who had both a CYP2C9 variant allele and taking a CYP2C9 interacting drug, TTR was 76.5% and 69.5% for the tecarfarin (n=24) and warfarin (n=31) groups, respectively (pint=0.24). This study included in 84 (14%) patients with a mechanical heart valve as an indication for anticoagulation therapy. No thrombotic or embolic events were observed in the tecarfarin treated subjects. In contrast to warfarin, tecarfarin is not affected by the cytochrome P450 inhibiting drug fluconazole, indicating a lower potential for interactions with other drugs.