
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Osmium is a chemical element; it has symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element. When experimentally measured using X-ray crystallography, it has a density of 22.59 g/cm3. Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

In the context of the periodic table a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter than elements that form metals and are often poor conductors of heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

The pnictogens are the chemical elements in group 15 of the periodic table. This group is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc).

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most [economically] important sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that contain only nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).

Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid H2WO4. It is a light yellow crystalline solid.
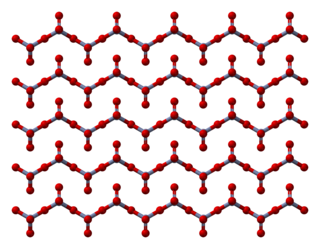
Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
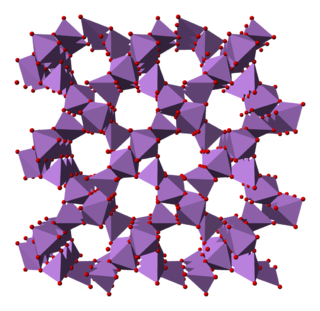
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.
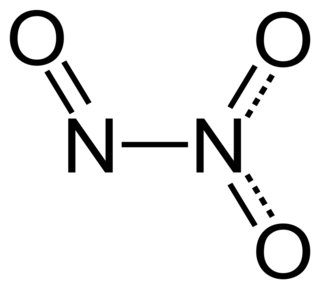
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
The chemical elements can be broadly divided into metals, metalloids, and nonmetals according to their shared physical and chemical properties. All elemental metals have a shiny appearance ; are good conductors of heat and electricity; form alloys with other metallic elements; and have at least one basic oxide. Metalloids are metallic-looking, often brittle solids that are either semiconductors or exist in semiconducting forms, and have amphoteric or weakly acidic oxides. Typical elemental nonmetals have a dull, coloured or colourless appearance; are often brittle when solid; are poor conductors of heat and electricity; and have acidic oxides. Most or some elements in each category share a range of other properties; a few elements have properties that are either anomalous given their category, or otherwise extraordinary.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.











