
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource.

Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th century by Joseph Aspdin, and is usually made from limestone. It is a fine powder, produced by heating limestone and clay minerals in a kiln to form clinker, grinding the clinker, and adding 2 to 3 percent of gypsum. Several types of portland cement are available. The most common, called ordinary portland cement (OPC), is grey, but white portland cement is also available. Its name is derived from its resemblance to Portland stone which is quarried on the Isle of Portland in Dorset, England. It was named by Joseph Aspdin who obtained a patent for it in 1824. His son William Aspdin is regarded as the inventor of "modern" portland cement due to his developments in the 1840s.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium.
Cement chemist notation (CCN) was developed to simplify the formulas cement chemists use on a daily basis. It is a shorthand way of writing the chemical formula of oxides of calcium, silicon, and various metals.

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.

Ye'elimite is the naturally occurring form of anhydrous calcium sulfoaluminate, Ca
4(AlO
2)
6SO
4. It gets its name from Har Ye'elim in Israel in the Hatrurim Basin west of the Dead Sea where it was first found in nature by Shulamit Gross, an Israeli mineralogist and geologist who studied the Hatrurim Formation.
Alite is an impure form of tricalcium silicate, Ca3SiO5, sometimes formulated as 3CaO·SiO2, typically with 3-4% of substituent oxides. It is the major, and characteristic, phase in Portland cement. The name was given by Törnebohm in 1897 to a crystal identified in microscopic investigation of Portland cement. Hatrurite is the name of a mineral that is substituted C3S.
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation).

Cement clinker is a solid material produced in the manufacture of portland cement as an intermediary product. Clinker occurs as lumps or nodules, usually 3 millimetres (0.12 in) to 25 millimetres (0.98 in) in diameter. It is produced by sintering limestone and aluminosilicate materials such as clay during the cement kiln stage.
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. It does not occur in nature, but is an important mineral phase in Portland cement.

Calcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
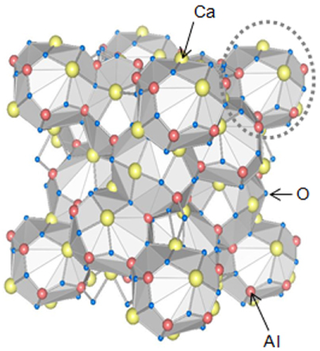
Dodecacalcium hepta-aluminate (12CaO·7Al2O3, Ca12Al14O33 or C12A7) is an inorganic solid that occurs rarely in nature as the mineral mayenite. It is an important phase in calcium aluminate cements and is an intermediate in the manufacture of Portland cement. Its composition and properties have been the subject of much debate, because of variations in composition that can arise during its high-temperature formation.
Monocalcium aluminate (CaAl2O4) is one of the series of calcium aluminates. It does occur in nature, although only very rarely, as two polymorphs known as krotite and dmitryivanovite, both from meteorites. It is important in the composition of calcium aluminate cements.

Calcium aluminoferrite is a dark brown crystalline phase commonly found in cements. In the cement industry it is termed tetra-calcium aluminoferrite or ferrite. In cement chemist notation (CCN), it is abbreviated as C
4AF meaning 4CaO·Al
2O
3·Fe
2O
3 in the oxide notation. It also exists in nature as the rare mineral brownmillerite.
An AFm phase is an "alumina, ferric oxide, monosubstituted" phase, or aluminate ferrite monosubstituted, or Al2O3, Fe2O3 mono, in cement chemist notation (CCN). AFm phases are important hydration products in the hydration of Portland cements and hydraulic cements.
Friedel's salt is an anion exchanger mineral belonging to the family of the layered double hydroxides (LDHs). It has affinity for anions as chloride and iodide and is capable of retaining them to a certain extent in its crystallographical structure.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damages are caused by the formation of expansive products produced by various chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms. Other damaging processes can also involve calcium leaching by water infiltration and different physical phenomena initiating cracks formation and propagation. All these detrimental processes and damaging agents adversely affects the concrete mechanical strength and its durability.
The pozzolanic activity is a measure for the degree of reaction over time or the reaction rate between a pozzolan and Ca2+ or calcium hydroxide (Ca(OH)2) in the presence of water. The rate of the pozzolanic reaction is dependent on the intrinsic characteristics of the pozzolan such as the specific surface area, the chemical composition and the active phase content.
AFt Phases refer to the calcium Aluminate Ferrite trisubstituted, or calcium aluminate trisubstituted, phases present in hydrated cement paste (HCP) in concrete.









