Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a "glass" for drinking, "glasses" for vision correction, and a "magnifying glass".

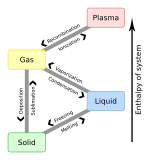
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates and Fermionic condensates, neutron-degenerate matter, and quark–gluon plasma.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
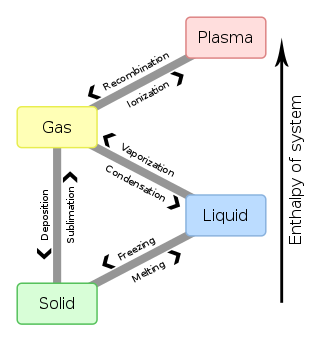
In physics, chemistry, and other related fields like biology, a phase transition is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment.

Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation.
In chemistry, the standard molar entropy is the entropy content of one mole of pure substance at a standard state of pressure and any temperature of interest. These are often chosen to be the standard temperature and pressure.

Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established defention, "freezing" means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. Per the established international definition, supercooling means ‘cooling a substance below the normal freezing point without solidification’ While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.
In thermodynamics, the entropy of fusion is the increase in entropy when melting a solid substance. This is almost always positive since the degree of disorder increases in the transition from an organized crystalline solid to the disorganized structure of a liquid; the only known exception is helium. It is denoted as and normally expressed in joules per mole-kelvin, J/(mol·K).

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.

A phase-change material (PCM) is a substance which releases/absorbs sufficient energy at phase transition to provide useful heat or cooling. Generally the transition will be from one of the first two fundamental states of matter - solid and liquid - to the other. The phase transition may also be between non-classical states of matter, such as the conformity of crystals, where the material goes from conforming to one crystalline structure to conforming to another, which may be a higher or lower energy state.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Thermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles. Data is expressed as temperature-dependent values for one mole of substance at the standard pressure of 101.325 kPa, or 100 kPa. Both of these definitions for the standard condition for pressure are in use.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
Melting-point depression is the phenomenon of reduction of the melting point of a material with a reduction of its size. This phenomenon is very prominent in nanoscale materials, which melt at temperatures hundreds of degrees lower than bulk materials.
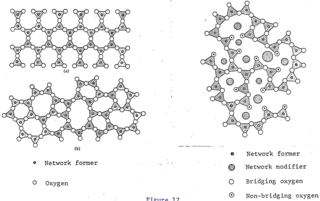
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.

In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure.
In condensed matter physics and physical chemistry, the terms viscous liquid, supercooled liquid, and glass forming liquid are often used interchangeably to designate liquids that are at the same time highly viscous, can be or are supercooled, and able to form a glass.
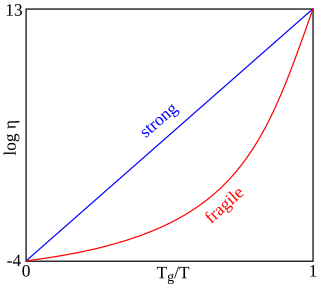
In glass sciences, fragility or "kinetic fragility" is a concept proposed by the Australian-American physical chemist C. Austen Angell. Fragility characterizes how rapidly the viscosity of a glass forming liquid approaches a very large value approximately 1012 Pa s during cooling. At this viscosity, the liquid is "frozen" into a solid and the corresponding temperature is known as the glass transition temperature Tg. Materials with a higher fragility have a more rapid increase in viscosity as approaching Tg, while those with a lower fragility have a slower increase in viscosity. Fragility is one of the most important concepts to understand viscous liquids and glasses. Fragility may be related to the presence of dynamical heterogeneity in glass forming liquids, as well as to the breakdown of the usual Stokes–Einstein relationship between viscosity and diffusion. Fragility has no direct relationship with the colloquial meaning of the word "fragility", which more closely relates to the brittleness of a material.