
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

Enthalpy is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work that was done against constant external pressure to establish the system's physical dimensions from to some final volume , i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.

Pressure is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure is the pressure relative to the ambient pressure.

In thermodynamics, the specific heat capacity of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as Massic heat capacity or as the Specific heat. More formally it is the heat capacity of a sample of the substance divided by the mass of the sample. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of 1 kg of water by 1 K is 4184 joules, so the specific heat capacity of water is 4184 J⋅kg−1⋅K−1.

Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density of a substance to the density of a given reference material. Specific gravity for solids and liquids is nearly always measured with respect to water at its densest ; for gases, the reference is air at room temperature. The term "relative density" is preferred in SI, whereas the term "specific gravity" is gradually being abandoned.
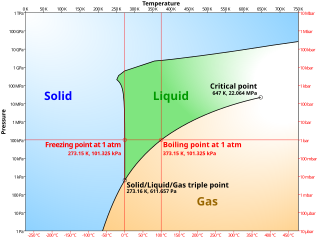
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.
In thermodynamics, the Joule–Thomson effect describes the temperature change of a real gas or liquid when it is expanding; typically caused by the pressure loss from flow through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This procedure is called a throttling process or Joule–Thomson process. The effect is purely an effect due to deviation from ideality, as any ideal gas has no JT effect.
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, and hydraulic motors that are assembled into hydraulic machinery, hydraulic drive systems, etc. In pneumatics, the working fluid is air or another gas which transfers force between pneumatic components such as compressors, vacuum pumps, pneumatic cylinders, and pneumatic motors. In pneumatic systems, the working gas also stores energy because it is compressible.
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas.
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is named after Rudolf Clausius and Benoît Paul Émile Clapeyron. However, this relation was in fact originally derived by Sadi Carnot in his Reflections on the Motive Power of Fire, which was published in 1824 but largely ignored until it was rediscovered by Clausius, Clapeyron, and Lord Kelvin decades later. Kelvin said of Carnot's argument that "nothing in the whole range of Natural Philosophy is more remarkable than the establishment of general laws by such a process of reasoning."

In thermodynamics, a critical point is the end point of a phase equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas comes into a supercritical phase, and so cannot be liquefied by pressure alone. At the critical point, defined by a critical temperatureTc and a critical pressurepc, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition in the absence of an external magnetic field.
The potential temperature of a parcel of fluid at pressure is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure , usually 1,000 hPa (1,000 mb). The potential temperature is denoted and, for a gas well-approximated as ideal, is given by

In thermodynamics, the compressibility factor (Z), also known as the compression factor or the gas deviation factor, describes the deviation of a real gas from ideal gas behaviour. It is simply defined as the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behaviour. In general, deviation from ideal behaviour becomes more significant the closer a gas is to a phase change, the lower the temperature or the larger the pressure. Compressibility factor values are usually obtained by calculation from equations of state (EOS), such as the virial equation which take compound-specific empirical constants as input. For a gas that is a mixture of two or more pure gases, the gas composition must be known before compressibility can be calculated.
Alternatively, the compressibility factor for specific gases can be read from generalized compressibility charts that plot as a function of pressure at constant temperature.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

In thermodynamics, vapor quality is the mass fraction in a saturated mixture that is vapor; in other words, saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. Vapor quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system. It has no meaning for substances which are not saturated mixtures . Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles. Working fluids can be classified by using the appearance of droplets in the vapor during the expansion step.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that transmits heat from one location at a certain temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or "cooler" if the objective is to cool the heat source. The operating principles in both cases are the same; energy is used to move heat from a colder place to a warmer place.

Gas is one of the four fundamental states of matter. The others are solid, liquid, and plasma. A pure gas may be made up of individual atoms, elemental molecules made from one type of atom, or compound molecules made from a variety of atoms. A gas mixture, such as air, contains a variety of pure gases. What distinguishes gases from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer.

In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law. The physical region covered by a system may or may not coincide with a control volume used to analyze the system.
A saturation dome is a graphical representation of the combination of vapor and gas that is used in thermodynamics. It can be used to find either the pressure or the specific volume as long as one already has at least one of these properties.

Non ideal compressible fluid dynamics (NICFD), or non ideal gas dynamics, is a branch of fluid mechanics studying the dynamic behavior of fluids not obeying ideal-gas thermodynamics. It is for example the case of dense vapors, supercritical flows and compressible two-phase flows. With the term dense vapors, we indicate all fluids in the gaseous state characterized by thermodynamic conditions close to saturation and the critical point. Supercritical fluids feature instead values of pressure and temperature larger than their critical values, whereas two-phase flows are characterized by the simultaneous presence of both liquid and gas phases.










