| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium oxide | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| ECHA InfoCard | 100.013.827 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
| UN number | 1825 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| Na2O | |||
| Molar mass | 61.979 g·mol−1 | ||
| Appearance | white solid | ||
| Density | 2.49 g/cm3 | ||
| Melting point | 1,132 °C (2,070 °F; 1,405 K) | ||
| Boiling point | 1,950 °C (3,540 °F; 2,220 K) sublimates | ||
| sublimates at 1275 °C | |||
| Reacts to form NaOH | |||
| Solubility | Reacts with ethanol | ||
| −19.8·10−6 cm3/mol | |||
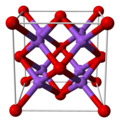
| Structure | |||
| Antifluorite (face centered cubic), cF12 | |||
| Fm3m, No. 225 | |||
| Tetrahedral (Na+); cubic (O2−) | |||
| Thermochemistry | |||
Heat capacity (C) | 72.95 J/(mol·K) | ||
Std molar entropy (S⦵298) | 73 J/(mol·K) [1] | ||
Std enthalpy of formation (ΔfH⦵298) | −416 kJ/mol [1] | ||
Gibbs free energy (ΔfG⦵) | −377.1 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | corrosive, reacts violently with water | ||
| GHS labelling: | |||
 [2] [2] | |||
| H314 | |||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | nonflammable | ||
| Safety data sheet (SDS) | ICSC 1653 | ||
| Related compounds | |||
Other anions | |||
Other cations | |||
Related compounds | Sodium hydroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component.