Related Research Articles
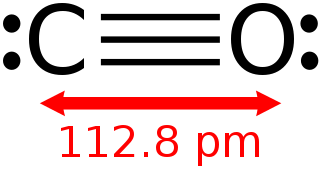
Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.
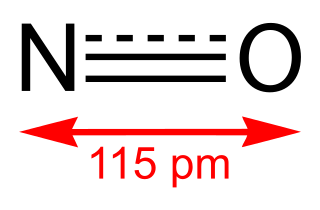
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is mainly a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO2−
3).
Lithium superoxide is an unstable inorganic salt with formula LiO2. A radical compound, it can be produced at low temperature in matrix isolation experiments, or in certain nonpolar, non-protic solvents. Lithium superoxide is also a transient species during the reduction of oxygen in a lithium–air galvanic cell, and serves as a main constraint on possible solvents for such a battery. For this reason, it has been investigated thoroughly using a variety of methods, both theoretical and spectroscopic.
Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important in the atmospheric reaction of bromine with ozone. It is similar to chlorine dioxide, the dioxide of its halogen neighbor one period higher on the periodic table.

Ethylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. It is an oxide of carbon, and can be described as the carbon-carbon covalent dimer of carbon monoxide. It can also be thought of as the dehydrated form of glyoxylic acid, or a ketone of ethenone H2C=C=O.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Disulfur monoxide or sulfur suboxide is an inorganic compound with the formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.
BigDFT is a free software package for physicists and chemists, distributed under the GNU General Public License, whose main program allows the total energy, charge density, and electronic structure of systems made of electrons and nuclei to be calculated within density functional theory (DFT), using pseudopotentials, and a wavelet basis.

Dioxidanylium, which is protonated molecular oxygen, or just protonated oxygen, is an ion with formula HO+
2. It is formed when hydrogen containing substances combust, and exists in the ionosphere, and in plasmas that contain oxygen and hydrogen. Oxidation by O2 in superacids could be by way of the production of protonated molecular oxygen.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
Erin Johnson is a Canadian computational chemist. She holds the Herzberg–Becke Chair at Dalhousie University. She works on density functional theory and intermolecular interactions.

Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.

John W. Birks is an American atmospheric chemist and entrepreneur who is best known for co-discovery with Paul Crutzen of the potential atmospheric effects of nuclear war known as nuclear winter. His most recent awards include the 2019 Haagen-Smit Clean Air Award for his contributions to atmospheric chemistry and the 2022 Future of Life Award for discovery of the nuclear winter effect.
References
- ↑ Stoll, Wolfgang (2011). "Thorium and Thorium Compounds". Ullmann's Encyclopedia of Industrial Chemistry . Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_001. ISBN 978-3527306732.
- ↑ Dewberry, Christopher T.; Etchison, Kerry C.; Cooke, Stephen A. (2007). "The pure rotational spectrum of the actinide-containing compound thorium monoxide". Physical Chemistry Chemical Physics. 9 (35): 4895–4897. Bibcode:2007PCCP....9.4895D. doi:10.1039/B709343H. PMID 17912418.
- ↑ Skripnikov, L. V. (2016-12-07). "Combined 4-component and relativistic pseudopotential study of ThO for the electron electric dipole moment search". The Journal of Chemical Physics. 145 (21): 214301. arXiv: 1610.00994 . Bibcode:2016JChPh.145u4301S. doi:10.1063/1.4968229. ISSN 0021-9606. PMID 28799403. S2CID 42337394.
- ↑ Denis, Malika; Fleig, Timo (2016-12-07). "In search of discrete symmetry violations beyond the standard model: Thorium monoxide reloaded". The Journal of Chemical Physics. 145 (21): 214307. Bibcode:2016JChPh.145u4307D. doi:10.1063/1.4968597. ISSN 0021-9606. PMID 28799357.
- ↑ Skripnikov, L. V.; Petrov, A. N.; Titov, A. V. (2013-12-14). "Communication: Theoretical study of ThO for the electron electric dipole moment search". The Journal of Chemical Physics. 139 (22): 221103. arXiv: 1308.0414 . Bibcode:2013JChPh.139v1103S. doi:10.1063/1.4843955. ISSN 0021-9606. PMID 24329049. S2CID 42153944.
- ↑ "The ACME EDM Experiment". electronedm.org. Retrieved 2018-08-16.
- ↑ He, Heming; Majewski, Jaroslaw; Allred, David D.; Wang, Peng; Wen, Xiaodong; Rector, Kirk D. (2017). "Formation of solid thorium monoxide at near-ambient conditions as observed by neutron reflectometry and interpreted by screened hybrid functional calculations". Journal of Nuclear Materials. 487: 288–296. Bibcode:2017JNuM..487..288H. doi: 10.1016/j.jnucmat.2016.12.046 .
- ↑ Hoch, Michael; Johnston, Herrick L. (1954). "The Reaction Occurring on Thoriated Cathodes". J. Am. Chem. Soc. 76 (19): 4833–4835. doi:10.1021/ja01648a018.