The actinide or actinoid series encompasses the 14 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive gray when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.

Zirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.
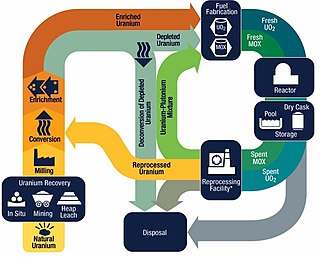
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.
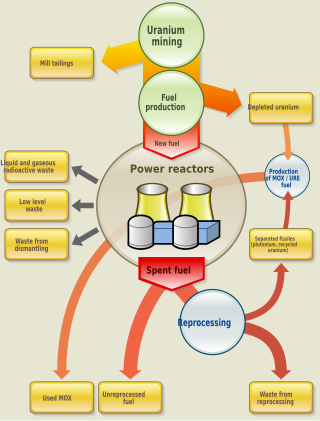
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.

The Bhabha Atomic Research Centre (BARC) is India's premier nuclear research facility, headquartered in Trombay, Mumbai, Maharashtra, India. It was founded by Homi Jehangir Bhabha as the Atomic Energy Establishment, Trombay (AEET) in January 1954 as a multidisciplinary research program essential for India's nuclear program. It operates under the Department of Atomic Energy (DAE), which is directly overseen by the Prime Minister of India.

In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. The heating element is usually an electrical filament heated by a separate electric current passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathodes rely on field electron emission or secondary electron emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a directly heated cathode, the filament is the cathode and emits the electrons. In an indirectly heated cathode, the filament or heater heats a separate metal cathode electrode which emits the electrons.

Uranium dioxide or uranium(IV) oxide , also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reactors. A mixture of uranium and plutonium dioxides is used as MOX fuel. Prior to 1960, it was used as yellow and black color in ceramic glazes and glass.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.

Environmental radioactivity is not limited to actinides; non-actinides such as radon and radium are of note. While all actinides are radioactive, there are a lot of actinides or actinide-relating minerals in the Earth's crust such as uranium and thorium. These minerals are helpful in many ways, such as carbon-dating, most detectors, X-rays, and more.
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous.
The liquid fluoride thorium reactor is a type of molten salt reactor. LFTRs use the thorium fuel cycle with a fluoride-based molten (liquid) salt for fuel. In a typical design, the liquid is pumped between a critical core and an external heat exchanger where the heat is transferred to a nonradioactive secondary salt. The secondary salt then transfers its heat to a steam turbine or closed-cycle gas turbine.

Cerium is a chemical element; it has symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.



















