In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.

In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions), which results in a compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

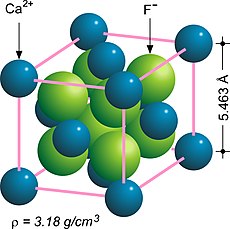
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.

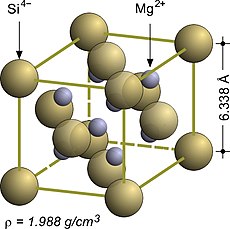
In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble in water. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.

Magnesium fluoride is an ionically bonded inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.
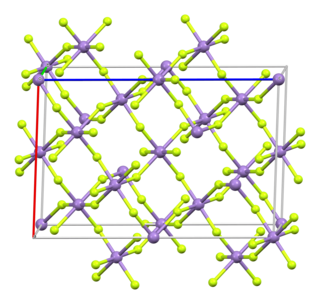
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.
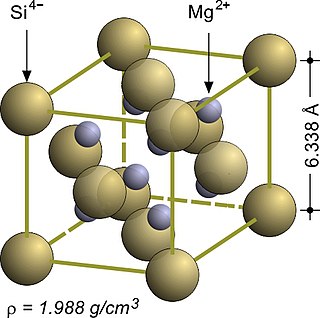
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As-grown Mg2Si usually forms black crystals; they are semiconductors with n-type conductivity and have potential applications in thermoelectric generators.

Ammonium fluoride is the inorganic compound with the formula NH4F. It crystallizes as small colourless prisms, having a sharp saline taste, and is highly soluble in water. Like all fluoride salts, it is moderately toxic in both acute and chronic overdose.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C.
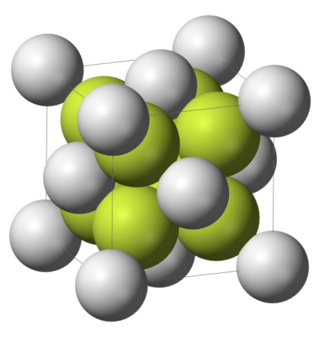
Difluorides are chemical compounds with two fluorine atoms per molecule.
The spinels are any of a class of minerals of general formulation AB
2X
4 which crystallise in the cubic (isometric) crystal system, with the X anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. Although the charges of A and B in the prototypical spinel structure are +2 and +3, respectively, other combinations incorporating divalent, trivalent, or tetravalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon, are also possible. The anion is normally oxygen; when other chalcogenides constitute the anion sublattice the structure is referred to as a thiospinel.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
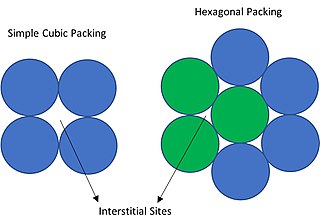
In crystallography, interstitial sites, holes or voids are the empty space that exists between the packing of atoms (spheres) in the crystal structure.
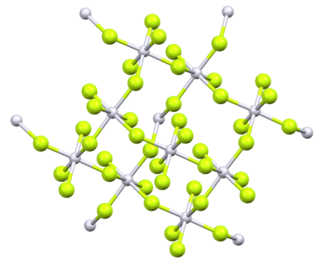
Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.

In crystallography, an anti-structure is obtained from a salt structure by exchanging anion and cation positions.

Ytterbium(II) fluoride is a binary inorganic compound of ytterbium and fluorine with the chemical formula YbF2.