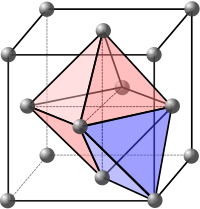
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.

A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect. Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization.

In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.

In geometry, a sphere packing is an arrangement of non-overlapping spheres within a containing space. The spheres considered are usually all of identical size, and the space is usually three-dimensional Euclidean space. However, sphere packing problems can be generalised to consider unequal spheres, spaces of other dimensions or to non-Euclidean spaces such as hyperbolic space.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement. Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is

Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite.
In condensed matter physics, the term geometrical frustration refers to a phenomenon where atoms tend to stick to non-trivial positions or where, on a regular crystal lattice, conflicting inter-atomic forces lead to quite complex structures. As a consequence of the frustration in the geometry or in the forces, a plenitude of distinct ground states may result at zero temperature, and usual thermal ordering may be suppressed at higher temperatures. Much studied examples are amorphous materials, glasses, or dilute magnets.
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).
In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity. In atomic systems, by convention, the APF is determined by assuming that atoms are rigid spheres. The radius of the spheres is taken to be the maximum value such that the atoms do not overlap. For one-component crystals (those that contain only one type of particle), the packing fraction is represented mathematically by

The tetrahedral-octahedral honeycomb, alternated cubic honeycomb is a quasiregular space-filling tessellation in Euclidean 3-space. It is composed of alternating regular octahedra and tetrahedra in a ratio of 1:2.
A Schottky defect is an excitation of the site occupations in a crystal lattice leading to point defects named after Walter H. Schottky. In ionic crystals, this defect forms when oppositely charged ions leave their lattice sites and become incorporated for instance at the surface, creating oppositely charged vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid.
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals.
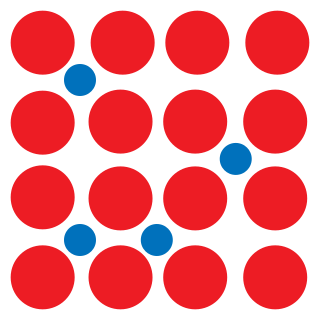
In materials science, an interstitial defect is a type of point crystallographic defect where an atom of the same or of a different type, occupies an interstitial site in the crystal structure. When the atom is of the same type as those already present they are known as a self-interstitial defect. Alternatively, small atoms in some crystals may occupy interstitial sites, such as hydrogen in palladium. Interstitials can be produced by bombarding a crystal with elementary particles having energy above the displacement threshold for that crystal, but they may also exist in small concentrations in thermodynamic equilibrium. The presence of interstitial defects can modify the physical and chemical properties of a material.

In crystallography, the hexagonal crystal family is one of the 6 crystal families, which includes two crystal systems and two lattice systems. While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent. In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice.
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids.
The spinels are any of a class of minerals of general formulation AB
2X
4 which crystallise in the cubic (isometric) crystal system, with the X anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. Although the charges of A and B in the prototypical spinel structure are +2 and +3, respectively, other combinations incorporating divalent, trivalent, or tetravalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon, are also possible. The anion is normally oxygen; when other chalcogenides constitute the anion sublattice the structure is referred to as a thiospinel.

Bicchulite has an ideal chemical formula of 2CaO•Al2O2•SiO2•H2O, which was formularized from the hydrothermal synthesis of synthetic gehlenite. Also, bicchulite was sighted in the mines of Japan with related minerals. This sodalite-type structured bicchulite has an uncommon ratio of aluminium to silicon, causing difficulties deciphering the structure. Because of bicchulite's structure it has a powdery texture, which leads to complications in obtaining information on the mineral's physical properties. Despite this problem, the color, specific gravity, and crystal size of bicchulite are known. Although bicchulite was only discovered about 40 years ago, technology has been rapidly advancing, allowing more accurate results to be made from experiments done today.

In crystallography, a stacking fault is a planar defect that can occur in crystalline materials. Crystalline materials form repeating patterns of layers of atoms. Errors can occur in the sequence of these layers and are known as stacking faults. Stacking faults are in a higher energy state which is quantified by the formation enthalpy per unit area called the stacking-fault energy. Stacking faults can arise during crystal growth or from plastic deformation. In addition, dislocations in low stacking-fault energy materials typically dissociate into an extended dislocation, which is a stacking fault bounded by partial dislocations.
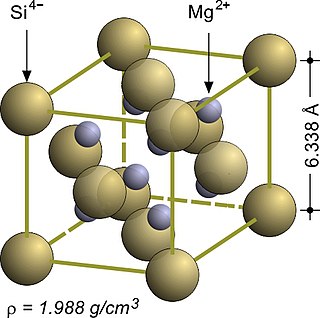
The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure.