Relation to other physical properties and units
| Quantity | Unit | SI value of unit |
|---|---|---|
| energy | eV | 1.602176634×10−19 J [1] |
| mass | eV/c2 | 1.78266192×10−36 kg |
| momentum | eV/c | 5.34428599×10−28 kg·m/s |
| temperature | eV/kB | 11604.51812 K |
| time | ħ/eV | 6.582119×10−16 s |
| distance | ħc/eV | 1.97327×10−7 m |
In the fields of physics in which the electronvolt is used, other quantities are typically measured using units derived from it; products with fundamental constants of importance in the theory are often used.
Mass
By mass–energy equivalence, the electronvolt corresponds to a unit of mass. It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/c2, where c is the speed of light in vacuum (from E = mc2). It is common to informally express mass in terms of eV as a unit of mass, effectively using a system of natural units with c set to 1. [3] The kilogram equivalent of 1 eV/c2 is:
For example, an electron and a positron, each with a mass of 0.511 MeV/c2, can annihilate to yield 1.022 MeV of energy. A proton has a mass of 0.938 GeV/c2. In general, the masses of all hadrons are of the order of 1 GeV/c2, which makes the GeV/c2 a convenient unit of mass for particle physics: [4]
The atomic mass constant (mu), one twelfth of the mass a carbon-12 atom, is close to the mass of a proton. To convert to electronvolt mass-equivalent, use the formula:
Momentum
By dividing a particle's kinetic energy in electronvolts by the fundamental constant c (the speed of light), one can describe the particle's momentum in units of eV/c. [5] In natural units in which the fundamental velocity constant c is numerically 1, the c may informally be omitted to express momentum using the unit electronvolt.

The energy–momentum relation in natural units (with ) is a Pythagorean equation. When a relatively high energy is applied to a particle with relatively low rest mass, it can be approximated as in high-energy physics such that an applied energy with expressed in the unit eV conveniently results in a numerically approximately equivalent change of momentum when expressed with the unit eV/c.
The dimension of momentum is T−1LM. The dimension of energy is T−2L2M. Dividing a unit of energy (such as eV) by a fundamental constant (such as the speed of light) that has the dimension of velocity (T−1L) facilitates the required conversion for using a unit of energy to quantify momentum.
For example, if the momentum p of an electron is 1 GeV/c, then the conversion to MKS system of units can be achieved by:
Distance
In particle physics, a system of natural units in which the speed of light in vacuum c and the reduced Planck constant ħ are dimensionless and equal to unity is widely used: c = ħ = 1. In these units, both distances and times are expressed in inverse energy units (while energy and mass are expressed in the same units, see mass–energy equivalence). In particular, particle scattering lengths are often presented using a unit of inverse particle mass.
Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following:
The above relations also allow expressing the mean lifetime τ of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via Γ = ħ/τ. For example, the B0
meson has a lifetime of 1.530(9) picoseconds, mean decay length is cτ = 459.7 μm, or a decay width of 4.302(25)×10−4 eV.
Conversely, the tiny meson mass differences responsible for meson oscillations are often expressed in the more convenient inverse picoseconds.
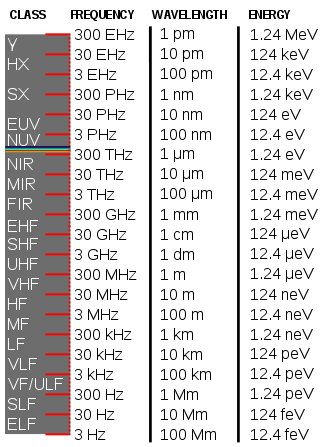
Energy in electronvolts is sometimes expressed through the wavelength of light with photons of the same energy:
Temperature
In certain fields, such as plasma physics, it is convenient to use the electronvolt to express temperature. The electronvolt is divided by the Boltzmann constant to convert to the Kelvin scale: where kB is the Boltzmann constant.
The kB is assumed when using the electronvolt to express temperature, for example, a typical magnetic confinement fusion plasma is 15 keV (kiloelectronvolt), which corresponds to 174 MK (megakelvin).
As an approximation: at a temperature of T = 20 °C, kBT is about 0.025 eV (≈ 290 K/11604 K/eV).
Wavelength


The energy E, frequency ν, and wavelength λ of a photon are related by where h is the Planck constant, c is the speed of light. This reduces to [6] A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV. Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm or frequency 241.8 THz.