
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current in a circuit is opposite to the direction of electron flow, so electrons flow out the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "-" (minus) is the anode.

Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode. They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen.
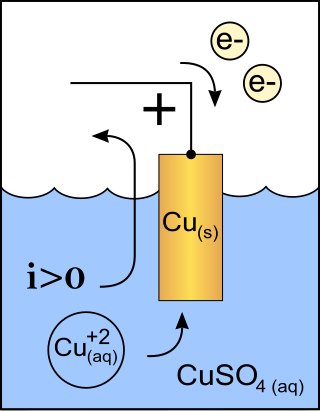
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode.

The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.

A triode is an electronic amplifying vacuum tube consisting of three electrodes inside an evacuated glass envelope: a heated filament or cathode, a grid, and a plate (anode). Developed from Lee De Forest's 1906 Audion, a partial vacuum tube that added a grid electrode to the thermionic diode, the triode was the first practical electronic amplifier and the ancestor of other types of vacuum tubes such as the tetrode and pentode. Its invention founded the electronics age, making possible amplified radio technology and long-distance telephony. Triodes were widely used in consumer electronics devices such as radios and televisions until the 1970s, when transistors replaced them. Today, their main remaining use is in high-power RF amplifiers in radio transmitters and industrial RF heating devices. In recent years there has been a resurgence in demand for low power triodes due to renewed interest in tube-type audio systems by audiophiles who prefer the pleasantly (warm) distorted sound of tube-based electronics.

A vacuum tube, electron tube, valve, or tube, is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied.
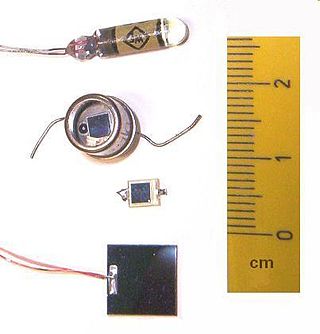
A photodiode is a light-sensitive semiconductor diode. It produces current when it absorbs photons.

A cold cathode is a cathode that is not electrically heated by a filament. A cathode may be considered "cold" if it emits more electrons than can be supplied by thermionic emission alone. It is used in gas-discharge lamps, such as neon lamps, discharge tubes, and some types of vacuum tube. The other type of cathode is a hot cathode, which is heated by electric current passing through a filament. A cold cathode does not necessarily operate at a low temperature: it is often heated to its operating temperature by other methods, such as the current passing from the cathode into the gas.

Photomultiplier tubes (photomultipliers or PMTs for short) are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum. They are members of the class of vacuum tubes, more specifically vacuum phototubes. These detectors multiply the current produced by incident light by as much as 100 million times or 108 (i.e., 160 dB), in multiple dynode stages, enabling (for example) individual photons to be detected when the incident flux of light is low.

A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.

A thyratron is a type of gas-filled tube used as a high-power electrical switch and controlled rectifier. Thyratrons can handle much greater currents than similar hard-vacuum tubes. Electron multiplication occurs when the gas becomes ionized, producing a phenomenon known as Townsend discharge. Gases used include mercury vapor, xenon, neon, and hydrogen. Unlike a vacuum tube (valve), a thyratron cannot be used to amplify signals linearly.

A photocathode is a surface engineered to convert light (photons) into electrons using the photoelectric effect. Photocathodes are important in accelerator physics where they are utilised in a photoinjector to generate high brightness electron beams. Electron beams generated with photocathodes are commonly used for free electron lasers and for ultrafast electron diffraction. Photocathodes are also commonly used as the negatively charged electrode in a light detection device such as a photomultiplier or phototube.
An image intensifier or image intensifier tube is a vacuum tube device for increasing the intensity of available light in an optical system to allow use under low-light conditions, such as at night, to facilitate visual imaging of low-light processes, such as fluorescence of materials in X-rays or gamma rays, or for conversion of non-visible light sources, such as near-infrared or short wave infrared to visible. They operate by converting photons of light into electrons, amplifying the electrons, and then converting the amplified electrons back into photons for viewing. They are used in devices such as night-vision goggles.

A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the striking voltage, the gas ionization becomes self-sustaining, and the tube glows with a colored light. The color depends on the gas used.

Photodetectors, also called photosensors, are sensors of light or other electromagnetic radiation. There is a wide variety of photodetectors which may be classified by mechanism of detection, such as photoelectric or photochemical effects, or by various performance metrics, such as spectral response. Semiconductor-based photodetectors typically photo detector have a p–n junction that converts light photons into current. The absorbed photons make electron–hole pairs in the depletion region. Photodiodes and photo transistors are a few examples of photo detectors. Solar cells convert some of the light energy absorbed into electrical energy.

The iconoscope was the first practical video camera tube to be used in early television cameras. The iconoscope produced a much stronger signal than earlier mechanical designs, and could be used under any well-lit conditions. This was the first fully electronic system to replace earlier cameras, which used special spotlights or spinning disks to capture light from a single very brightly lit spot.

Albert Wallace Hull was an American physicist and electrical engineer who made contributions to the development of vacuum tubes, and invented the magnetron. He was a member of the National Academy of Sciences.

In electromagnetism, the Townsend discharge or Townsend avalanche is a ionisation process for gases where free electrons are accelerated by an electric field, collide with gas molecules, and consequently free additional electrons. Those electrons are in turn accelerated and free additional electrons. The result is an avalanche multiplication that permits electrical conduction through the gas. The discharge requires a source of free electrons and a significant electric field; without both, the phenomenon does not occur.
Mellon optical memory was an early form of computer memory invented at the Mellon Institute in 1951. The device used a combination of photoemissive and phosphorescent materials to produce a "light loop" between two surfaces. The presence or lack of light, detected by a photocell, represented a one or zero. Although promising, the system was rendered obsolete with the introduction of magnetic-core memory in the early 1950s. It appears that the system was never used in production.
















