The actinide or actinoid series encompasses the 14 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive gray when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.

Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula HO−C(=O)−C(=O)−OH, also written as (COOH)2 or (CO2H)2 or H2C2O4. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
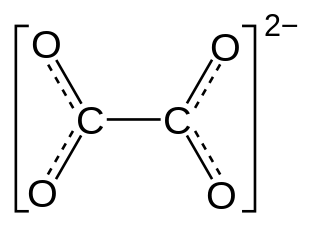
Oxalate is an anion with the chemical formula formula C2O2−4. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate, and several esters such as dimethyl oxalate. It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.

Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium.

Uranyl nitrate is a water-soluble yellow uranium salt with the formula UO2(NO3)2 · n H2O. The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels.

Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored salts.

Thorium(IV) chloride describes a family of inorganic compounds with the formula ThCl4(H2O)n. Both the anhydrous and tetrahydrate (n = 4) forms are known. They are hygroscopic, water-soluble white salts.

Isosaccharinic acid (ISA) is a six-carbon sugar acid which is formed by the action of calcium hydroxide on lactose and other carbohydrates. It is of interest because it may form in intermediate-level nuclear waste stores when cellulose is degraded by the calcium hydroxide in cements such as Portland cement. The calcium salt of the alpha form of ISA is very crystalline and quite insoluble in cold water, but in hot water it is soluble.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Sodium hydrogenoxalate is salt of formula NaHC
2O
4, consisting of sodium cations Na+
and hydrogenoxalate anions HC
2O−
4 or HO(O=)C-C(=O)O−
. The anion can be described as the result of removing one hydrogen ion H+
from oxalic acid H
2C
2O
4, or adding one to the oxalate anion C
2O2−
4.

Caesium oxalate (standard IUPAC spelling) dicesium oxalate, or cesium oxalate (American spelling) is the oxalate of caesium. Caesium oxalate has the chemical formula of Cs2C2O4.
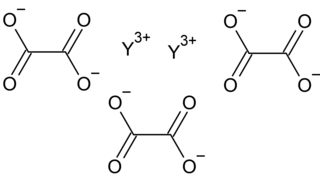
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.
The carbonate oxalates are mixed anion compounds that contain both carbonate (CO3) and oxalate (C2O4) anions. Most compounds incorporate large trivalent metal ions, such as the rare earth elements. Some carbonate oxalate compounds of variable composition are formed by heating oxalates.
Neptunium (IV) oxalate is an inorganic compound, a salt of neptunium and oxalic acid with the chemical formula Np(C2O4)2. The compound is slightly soluble in water, forms crystalline hydrates—green crystals.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Neptunium compounds are compounds containg the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.















