
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".

An actinometer is an instrument that can measure the heating power of radiation. Actinometers are used in meteorology to measure solar radiation as pyranometers, pyrheliometers and net radiometers.

Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.
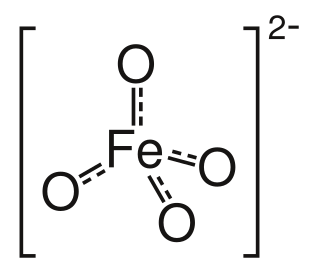
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.

A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula MIMIII(SO4)2·12H2O) and Tutton's salts (with the general formula (MI)2MII(SO4)2·6H2O). Other examples include potassium sodium tartrate, ammonium iron(II) sulfate (Mohr's salt), potassium uranyl sulfate (used to discover radioactivity) and bromlite BaCa(CO3)2. The fluorocarbonates contain fluoride and carbonate anions. Many coordination complexes form double salts.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

Croconic acid is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.

Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water.

Sodium ferrioxalate are inorganic compounds with the formula Na3Fe(C2O4)3(H2O)n. The pentahydrate has been characterized by X-ray crystallography. In contrast the potassium, ammonium, and rubidium salts crystallize from water as their trihydrates.

Ferric ammonium oxalate is the ammonium salt of the anionic trisoxalato coordination complex of iron(III). It is a precursor to iron oxides, diverse coordination polymers, and Prussian Blue. The latter behavior is relevant to the manufacture of blueprint paper. Ferric ammonium oxalate has also been used in the synthesis of superconducting salts with bis(ethylene)dithiotetrathiafulvalene (BEDT-TTF), see Organic superconductor.

Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe2(C2O4)3(H2O)x but could also refer to salts of [Fe(C2O4)3]3-. Fe2(C2O4)3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula [Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. This article emphasizes the coordination polymers.
Potassium ferrooxalate, also known as potassium bisoxalatoferrate(II), is a salt with the formula K2Fe(C2O4)2(H2O)x. The anion is a transition metal oxalate complex, consisting of an atom of iron in the +2 oxidation state bound to oxalate (C
2O2−
4) ligands and water.

Hydromelonic acid, is an elusive chemical compound with formula C
9H
3N
13 or (HNCN)
3(C
6N
7), whose molecule would consist of a heptazine H3(C
6N
7) molecule, with three cyanamido groups H–N=C=N– or N≡C–NH– substituted for the hydrogen atoms.
The borate oxalates are chemical compounds containing borate and oxalate anions. Where the oxalate group is bound to the borate via oxygen, a more condensed anion is formed that balances less cations. These can be termed boro-oxalates, bis(oxalato)borates, or oxalatoborates or oxalate borates. The oxalatoborates are heterocyclic compounds with a ring containing -O-B-O-. Bis(oxalato)borates are spiro compounds with rings joined at the boron atom.

Transition metal oxalate complexes are coordination complexes with oxalate (C2O42−) ligands. Some are useful commercially, but the topic has attracted regular scholarly scrutiny. Oxalate (C2O42-) is a kind of dicarboxylate ligand. As a small, symmetrical dinegative ion, oxalate commonly forms five-membered MO2C2 chelate rings. Mixed ligand complexes are known, e.g., [Co(C2O4)(NH3)4]κ+.
An oxalate chloride or oxalato chloride is a mixed anion compound contains both oxalate and chloride anions.


















