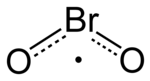
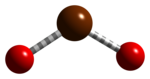
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).

Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium.

Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O. The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Plutonium(IV) fluoride is a chemical compound with the formula (PuF4). This salt is generally a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants. Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.

Iodine oxides are chemical compounds of oxygen and iodine. Iodine has only two stable oxides which are isolatable in bulk, iodine tetroxide and iodine pentoxide, but a number of other oxides are formed in trace quantities or have been hypothesized to exist. The chemistry of these compounds is complicated with only a few having been well characterized. Many have been detected in the atmosphere and are believed to be particularly important in the marine boundary layer.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.

Mercury(II) bromide or mercuric bromide is an inorganic compound with the formula HgBr2. This white solid is a laboratory reagent. Like all mercury salts, it is highly toxic.
Mercury(I) bromide or mercurous bromide is the chemical compound composed of mercury and bromine with the formula Hg2Br2. It changes color from white to yellow when heated and fluoresces a salmon color when exposed to ultraviolet light. It has applications in acousto-optical devices.

Rhenium(IV) oxide or rhenium dioxide is the inorganic compound with the formula ReO2. This gray to black crystalline solid is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this chemical.
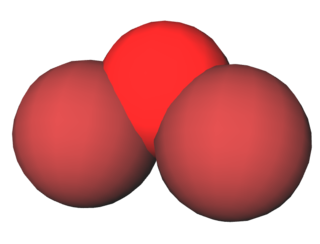
Dibromine monoxide is the chemical compound composed of bromine and oxygen with the formula Br2O. It is a dark brown solid which is stable below −40 °C and is used in bromination reactions. It is similar to dichlorine monoxide, the monoxide of its halogen neighbor one period higher on the periodic table. The molecule is bent, with C2v molecular symmetry. The Br−O bond length is 1.85 Å and the Br−O−Br bond angle is 112°, similar to dichlorine monoxide.

Dibromine pentoxide is the chemical compound composed of bromine and oxygen with the formula Br2O5. It is a colorless solid that is stable below −20 °C. It has the structure O2Br−O−BrO2, the Br−O−Br bond is bent with bond angle 121.2°. Each BrO3 group is pyramidal with the bromine atom at the apex.

Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula Br2O3. It is an orange solid that is stable below −40 °C. It has the structure Br−O−BrO2 (bromine bromate). It was discovered at 1993. The bond angle of Br−O−Br is 111.7°, the bond angle of O−Br=O is 103.1°, and the bond angle of O=Br=O is 107.6°. The Br−OBrO2 bond length is 1.845Å, the O−BrO2 bond length is 1.855Å, and the Br=O bond length is 1.612Å.

Bromine monoxide is a binary inorganic compound of bromine and oxygen with the chemical formula BrO. A free radical, this compound is the simplest of many bromine oxides. The compound is capable of influencing atmospheric chemical processes. Naturally, BrO can be found in volcanic plumes. BrO is similar to the oxygen monofluoride, chlorine monoxide and iodine monoxide radicals.
Iodine trifluoride dioxide is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F3. The compound was first obtained by Engelbrecht and Petersy in 1969.
Chlorine trifluoride dioxide is an inorganic compound of chlorine, fluorine, and oxygen with the chemical formula ClO2F3.