
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered, even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source.
Unconventional superconductors are materials that display superconductivity which is not explained by the usual BCS theory or its extension, the Eliashberg theory. The pairing in unconventional superconductors may originate from some other mechanism than the electron–phonon interaction. Alternatively, a superconductor is unconventional if the superconducting order parameter transforms according to a non-trivial irreducible representation of the point group or space group of the system. Per definition, superconductors that break additional symmetries to U (1) symmetry are known as unconventional superconductors.

High-temperature superconductivity is superconductivity in materials with a critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first breakthrough of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.

Yttrium arsenide is an inorganic compound of yttrium and arsenic with the chemical formula YAs. It can be prepared by reacting yttrium and arsenic at high temperature. Some literature has done research on the eutectic system of it and zinc arsenide.
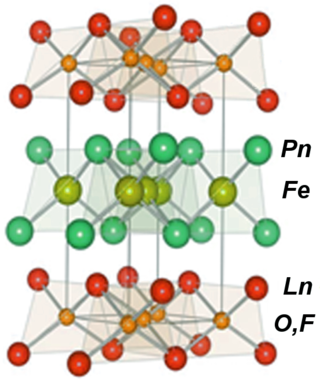
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..

The 122 iron arsenide unconventional superconductors are part of a new class of iron-based superconductors. They form in the tetragonal I4/mmm, ThCr2Si2 type, crystal structure. The shorthand name "122" comes from their stoichiometry; the 122s have the chemical formula AEFe2Pn2, where AE stands for alkaline earth metal (Ca, Ba Sr or Eu) and Pn is pnictide (As, P, etc.). These materials become superconducting under pressure and also upon doping. The maximum superconducting transition temperature found to date is 38 K in the Ba0.6K0.4Fe2As2. The microscopic description of superconductivity in the 122s is yet unclear.
Superstripes is a generic name for a phase with spatial broken symmetry that favors the onset of superconducting or superfluid quantum order. This scenario emerged in the 1990s when non-homogeneous metallic heterostructures at the atomic limit with a broken spatial symmetry have been found to favor superconductivity. Before a broken spatial symmetry was expected to compete and suppress the superconducting order. The driving mechanism for the amplification of the superconductivity critical temperature in superstripes matter has been proposed to be the shape resonance in the energy gap parameters ∆n that is a type of Fano resonance for coexisting condensates.

In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements.
Iron(II) selenide refers to a number of inorganic compounds of ferrous iron and selenide (Se2−). The phase diagram of the system Fe–Se reveals the existence of several non-stoichiometric phases between ~49 at. % Se and ~53 at. % Fe, and temperatures up to ~450 °C. The low temperature stable phases are the tetragonal PbO-structure (P4/nmm) β-Fe1−xSe and α-Fe7Se8. The high temperature phase is the hexagonal, NiAs structure (P63/mmc) δ-Fe1−xSe. Iron(II) selenide occurs naturally as the NiAs-structure mineral achavalite.
Kathryn Ann Moler is an American physicist, and current dean of research at Stanford University. She received her BSc (1988) and Ph.D. (1995) from Stanford University. After working as a visiting scientist at IBM T.J. Watson Research Center in 1995, she held a postdoctoral position at Princeton University from 1995 to 1998. She joined the faculty of Stanford University in 1998, and became an Associate in CIFAR's Superconductivity Program in 2000. She became an associate professor at Stanford in 2002 and is currently a professor of applied physics and of Physics at Stanford. She currently works in the Geballe Laboratory for Advanced Materials (GLAM), and is the director of the Center for Probing the Nanoscale (CPN), a National Science Foundation-funded center where Stanford and IBM scientists continue to improve scanning probe methods for measuring, imaging, and controlling nanoscale phenomena. She lists her scientific interests and main areas of research and experimentation as:

Hideo Hosono is a Japanese material scientist most known for the discovery of iron-based superconductors.
Chen Xianhui is a Chinese physicist. He is a Changjiang professor of physics of the University of Science and Technology of China (USTC). He was elected an academician of the Chinese Academy of Sciences (CAS) in 2015 and is known for his breakthroughs on iron-based superconductors. He won the State Natural Science Award with Zhao Zhongxian and others in 2013 and the Bernd T. Matthias Prize for Superconducting Materials in 2015. His research is mainly on experimental condensed matter physics and materials science.
Oxyphosphides are chemical compounds formally containing the group PO, with one phosphorus and one oxygen atom. The phosphorus and oxygen are not bound together as in phosphates or phosphine oxides, instead they are bound separately to the cations (metals), and could be considered as a mixed phosphide-oxide compound. So a compound with OmPn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxy-pnictide compounds.
Suchitra Sebastian is a condensed matter physicist at Cavendish Laboratory, University of Cambridge. She is known for her discoveries of exotic quantum phenomena that emerge in complex materials. In particular, she is known for the discovery of unconventional insulating materials which display simultaneous conduction-like behaviour. In 2022 she was awarded the New Horizons in Physics Prize by the Breakthrough Foundation. She was named as one of thirty Exceptional Young Scientists by the World Economic Forum in 2013, one of The Next Big Names in Physics by the Financial Times in 2013, and spoke at the World Economic Forum at Davos in 2016.
Oxyarsenides or arsenide oxides are chemical compounds formally containing the group AsO, with one arsenic and one oxygen atom. The arsenic and oxygen are not bound together as in arsenates or arsenites, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed arsenide-oxide compound. So a compound with OmAsn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.
Oxybismuthides or bismuthide oxides are chemical compounds formally containing the group BiO, with one bismuth and one oxygen atom. The bismuth and oxygen are not bound together as in bismuthates, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed bismuthide-oxide compound. So a compound with OmBin requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.
An arsenide hydride or hydride arsenide is a chemical compound containing hydride (H−) and arsenide (As3−) ions in a single phase. They are in the class of mixed anion compounds.

Arsenide nitrides or nitride arsenides are compounds containing anions composed of nitride (N3−) and arsenide (As3−). They can be considered as mixed anion compounds or mixed pnictide compounds. Related compounds include the arsenide phosphides, germanide arsenides, arsenide carbides, and phosphide nitrides.
Pengcheng Dai is a Chinese American experimental physicist and academic. He is the Sam and Helen Worden Professor of Physics in the Department of Physics and Astronomy at Rice University.







