 | |
 | |
| Names | |
|---|---|
| IUPAC names Manganese dioxide Manganese(IV) oxide | |
| Other names Pyrolusite, hyperoxide of manganese, black oxide of manganese, manganic oxide | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.821 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| MnO 2 | |
| Molar mass | 86.9368 g/mol |
| Appearance | Brown-black solid |
| Density | 5.026 g/cm3 |
| Melting point | 535 °C (995 °F; 808 K) (decomposes) |
| Insoluble | |
| +2280.0×10−6 cm3/mol [1] | |
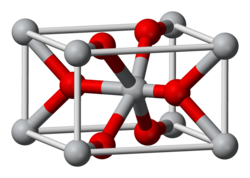
| Structure [2] | |
| Tetragonal, tP6, No. 136 | |
| P42/mnm | |
a = 0.44008 nm, b = 0.44008 nm, c = 0.28745 nm | |
Formula units (Z) | 2 |
| Thermochemistry [3] | |
Heat capacity (C) | 54.1 J·mol−1·K−1 |
Std molar entropy (S⦵298) | 53.1 J·mol−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) | −520.0 kJ·mol−1 |
Gibbs free energy (ΔfG⦵) | −465.1 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H332 | |
| P261, P264, P270, P271, P301+P312, P304+P312, P304+P340, P312, P330, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 535 °C (995 °F; 808 K) |
| Safety data sheet (SDS) | ICSC 0175 |
| Related compounds | |
Other anions | Manganese disulfide |
Other cations | Technetium dioxide Rhenium dioxide |
| Manganese(II) oxide Manganese(II,III) oxide Manganese(III) oxide Manganese heptoxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery, although it is also used for other battery chemistries such as aqueous zinc-ion batteries. [4] [5] MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4 . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries. [6] [7]
