
An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as [[potassium permanganate|KMnO
4]]. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.
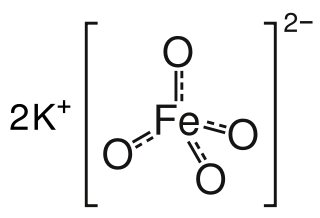
Potassium ferrate is the chemical compound with the formula K2FeO4. This purple salt is paramagnetic, and is a rare example of an iron(VI) compound. In most of its compounds, iron has the oxidation state +2 or +3 (Fe2+ or Fe3+). Reflecting its high oxidation state, FeO2−4 is a powerful oxidizing agent.

A permanganate is a chemical compound containing the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a transition metal oxo complex with tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized. For example, trichloroethane (C2H3Cl3) is oxidized by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process:

In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds.

Sodium manganate is the inorganic compound with the formula Na2MnO4. This deep green solid is a rarely encountered analogue of the related salt K2MnO4. Sodium manganate is rare because it cannot be readily prepared from the oxidation of manganese dioxide and sodium hydroxide. Instead this oxidation reaction tends to stop at producing sodium hypomanganate, Na3MnO4, and even this Mn(V) salt is unstable in solution. Sodium manganate can be produced by reduction of sodium permanganate under basic conditions:
Potassium manganate is the inorganic compound with the formula K2MnO4. This green-colored salt is an intermediate in the industrial synthesis of potassium permanganate, a common chemical. Occasionally, potassium manganate and potassium permanganate are confused, but these compounds's properties are distinct.
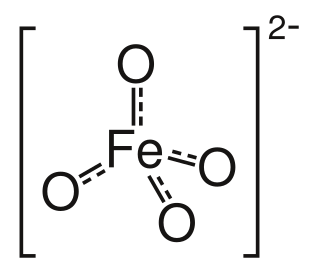
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH.
In chemistry, hypomanganate, also called manganate(V) or tetraoxidomanganate(3−), is a trivalent anion (negative ion) composed of manganese and oxygen, with formula MnO3−
4.

Manganese(II) sulfate usually refers to the inorganic compound with the formula MnSO4·H2O. This pale pink deliquescent solid is a commercially significant manganese(II) salt. Approximately 260,000 tonnes of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds. Manganese-deficient soil is remediated with this salt.

Sodium permanganate is the inorganic compound with the formula NaMnO4. It is closely related to the more commonly encountered potassium permanganate, but it is generally less desirable, because it is more expensive to produce. It is mainly available as the monohydrate. This salt absorbs water from the atmosphere and has a low melting point. Being about 15 times more soluble than KMnO4, sodium permanganate finds some applications where very high concentrations of MnO4− are sought.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.
Permanganometry is one of the techniques used in chemical quantitative analysis. It is a redox titration that involves the use of permanganates to measure the amount of analyte present in unknown chemical samples. It involves two steps, namely the titration of the analyte with potassium permanganate solution and then the standardization of potassium permanganate solution with standard sodium oxalate solution. The titration involves volumetric manipulations to prepare the analyte solutions.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.

Barium manganate is an inorganic compound with the formula BaMnO4. It is used as an oxidant in organic chemistry. It belongs to a class of compounds known as manganates in which the manganese resides in a +6 oxidation state. Manganate should not to be confused with permanganate which contains manganese(VII). Barium manganate is a powerful oxidant, popular in organic synthesis and can be used in a wide variety of oxidation reactions.
Barium permanganate is a chemical compound, with the formula Ba(MnO4)2. It forms violet to brown crystals that are sparingly soluble in water.
The chemical chameleon is a redox reaction, well known from classroom demonstrations, that exploits the dramatic color changes associated with the various oxidation states of manganese.














