 | |
| Names | |
|---|---|
| IUPAC name Potassium peroxysulfate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.158 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
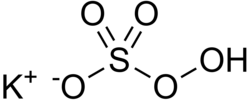
| KHSO5 | |
| Molar mass | 152.2 g/mol |
| Appearance | white solid |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Oxidant, corrosive |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ChemicalBook.com SDS [1] |
| Related compounds | |
Related compounds | Potassium persulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium peroxymonosulfate, also referred to as potassium peroxysulfate and potassium monopersulfate (KMPS), is an inorganic compound with the formula KHSO5. It is the mono-potassium salt derived from peroxymonosulfuric acid (Caro's acid). It is a constituent of the widely used oxidizing agent called Oxone, which is a triple salt with the formula 2KHSO5·KHSO4·K2SO4. [2] [3] [4]
