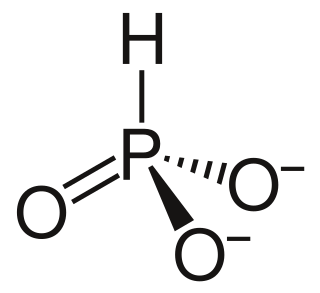
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.

Potassium alum, potash alum, or potassium aluminium sulfate is a chemical compound first mentioned under various Sanskrit names in Ayurvedic medicinal texts such as charak samhita, sushrut samhita, and ashtang hridaya; is chemically defined as the double sulfate of potassium and aluminium, with chemical formula KAl(SO4)2. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution and cubic structure in an alkali solution with space group Pa3 and lattice parameter of 12.18 Å. The compound is the most important member of the generic class of compounds called alums, and is often called simply alum.

Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent. When dissolved in water it forms sodium bisulfite.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.
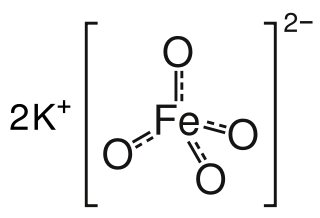
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.
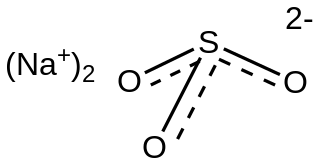
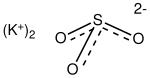
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. It is also suitable for the softening of lignin in the pulping and refining processes of wood and lignocellulosic materials. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Potassium superoxide is an inorganic compound with the formula KO2. It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a CO2 scrubber, H2O dehumidifier, and O2 generator in rebreathers, spacecraft, submarines, and spacesuits.
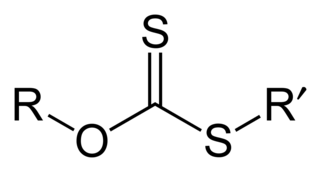
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.
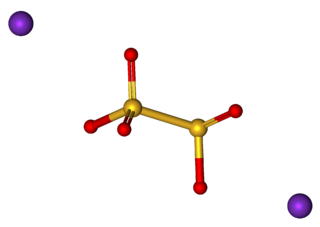
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure.
Potassium persulfate is the inorganic compound with the formula K2S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, commonly used to initiate polymerizations.

Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximately correctly mentioned formula chemical formula KHSO3. Potassium bisulfite in fact is not an actual compound, but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5.

Potassium chlorochromate is an inorganic compound with the formula KCrO3Cl. It is the potassium salt of chlorochromate, [CrO3Cl]−. It is a water-soluble orange compound is used occasionally for oxidation of organic compounds. It is sometimes called Péligot's salt, in recognition of its discoverer Eugène-Melchior Péligot.

A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO−
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such as a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.
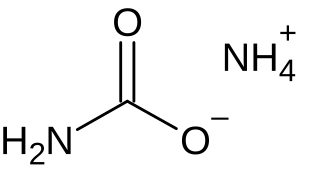
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.

Chevreul's salt (copper(I,II) sulfite dihydrate, Cu2SO3•CuSO3•2H2O or Cu3(SO3)2•2H2O), is a copper salt which was prepared for the first time by a French chemist Michel Eugène Chevreul in 1812. Its unusual property is that it contains copper in both of its common oxidation states, making it a mixed-valence complex. It is insoluble in water and stable in air. What was known as Rogojski's salt is a mixture of Chevreul's salt and metallic copper.
Nickel dicyanide is the inorganic compound with a chemical formula Ni(CN)2. It is a gray-green solid that is insoluble in most solvents.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.

Potassium tetracyanonickelate (IUPAC: Potassium tetracyanido nickelate(II)) is the inorganic compound with the formula K2Ni(CN)4. It is usually encountered as the monohydrate but the anhydrous salt is also known. Both are yellow, water-soluble, diamagnetic solids. The salt consists of potassium ions and the tetracyanonickelate coordination complex, which is square planar. The [Ni(CN)4]2- anions are arranged in a columnar structure with Ni---Ni distances of 4.294 Å, which is well beyond the sum of the van der Waals radius of the nickel cation. This columnar structure resembles those of the other [M(CN)4]2- anions of the heavy congeners of the group 10 metals (M = Pd, Pt).