 | |
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.021.173 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1485 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
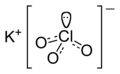
| KClO3 | |
| Molar mass | 122.55 g·mol−1 |
| Appearance | white crystals or powder |
| Density | 2.32 g/cm3 |
| Melting point | 356 °C (673 °F; 629 K) |
| Boiling point | 400 °C (752 °F; 673 K) decomposes [1] |
| |
| Solubility | negligible in acetone and liquid ammonia [1] |
| Solubility in glycerol | 1 g/100g (20 °C (68 °F; 293 K)) [1] |
| −43.8×10−6 cm3/mol | |
Refractive index (nD) | 1.40835 |
| Structure | |
| monoclinic | |
| Thermochemistry [1] | |
Heat capacity (C) | −391.2 J/(mol·K) |
Std molar entropy (S⦵298) | 142.97 J/(mol·K) [3] |
Std enthalpy of formation (ΔfH⦵298) | −391.2 kJ/mol [3] |
Gibbs free energy (ΔfG⦵) | −289.9 kJ/mol |
| Hazards | |
| GHS labelling: [4] | |
  | |
| Danger | |
| H271, H301, H401 [4] | |
| P210, P220, P221, P264, P270, P273, P280, P283, P301+P310+P330, P306+P360, P370+P378, P371+P380+P375, P405, P501 [4] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 100 mg/kg (oral, rat) [5] |
LC50 (median concentration) | >5.1 mg/L [5] |
| Related compounds | |
Other anions | |
Other cations | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium chlorate is the inorganic compound with the molecular formula KClO3. In its pure form, it is a white solid. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. [6]


