
Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
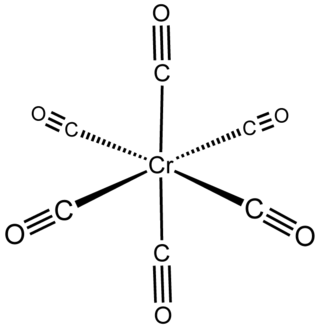
Chromium hexacarbonyl is a chromium(0) organometallic compound with the formula Cr(CO)6. It is a homoleptic complex, which means that all the ligands are identical. It is a colorless crystalline air-stable solid, with a high vapor pressure.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic diene with the formula C5(CH3)5H, often written C5Me5H, where Me is CH3. It is a colorless liquid.
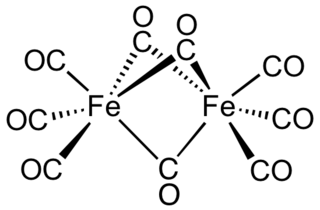
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use.

Bromopentacarbonylrhenium(I) is an inorganic compound of rhenium, commonly used for the syntheses of other rhenium complexes.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
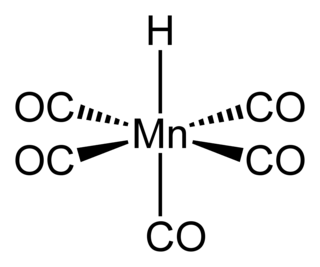
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene).

Xylylene dibromide is an organic compound with the formula C6H4(CH2Br)2. It is an off-white solid that, like other benzyl halides, a strong lachrymator. It is a useful reagent owing to the convenient reactivity of the two C-Br bonds. Two other isomers are known, para- and meta-xylylene dibromide.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.



















