
The Grignard reaction is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides are added to the carbonyl groups of either an aldehyde or ketone under anhydrous conditions. This reaction is important for the formation of carbon–carbon bonds.

Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate () with an organic halide or pseudohalide () to form a new alkane, as well as an ill-defined organocopper species and lithium (pseudo)halide as byproducts.
The Sakurai reaction is the chemical reaction of carbon electrophiles with allyltrimethylsilane catalyzed by strong Lewis acids. The reaction achieves results similar to the addition of an allyl Grignard reagent to the carbonyl.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.

1-Bromohexane is organobromine compound with formula Br(CH2)5CH3. It is a colorless liquid.
A carbometallation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometallation.
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6H5SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these similarities. Phenylsilane is soluble in organic solvents.
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles. Organocopper reagents are now commonly used in organic synthesis as mild, selective nucleophiles for substitution and conjugate addition reactions.
Cyclopropanol is an organic compound with the chemical formula C3H6O. It contains a cyclopropyl group with a hydroxyl group attached to it. The compound is highly unstable due to the three-membered ring, and is susceptible to reactions that open the ring. It is highly prone to rearrangement, undergoing structural isomerization to form propanal. This property is useful synthetically: cyclopropanol can be used as a synthon for the homoenolate of propanal. The chemical is also useful as a reagent to introduce a cyclopropyl group into ester, sulfate, and amine linkages. The resulting cyclopropyl-containing compounds have been used in investigations of potential antiviral drugs and of modulators of protein trafficking.

Marjorie Constance Caserio was an English chemist. In 1975, she was awarded the Garvan Medal by the American Chemical Society.
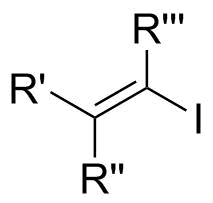
In organic chemistry, a vinyl iodide functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross-coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and Suzuki coupling. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective synthesis of natural products and drugs.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).

The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols.
Chlorocyclopropane is a organochlorine compound with the chemical formula C3H5Cl. The compound is a member of haloalkane family.











