
Xenon hexafluoroplatinate is the product of the reaction of platinum hexafluoride with xenon, in an experiment that proved the chemical reactivity of the noble gases. This experiment was performed by Neil Bartlett at the University of British Columbia, who formulated the product as "Xe+[PtF6]−", although subsequent work suggests that Bartlett's product was probably a salt mixture and did not in fact contain this specific salt.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 8 or 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

Iodine heptafluoride is an interhalogen compound with the chemical formula IF7. It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a heptacoordinated system.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
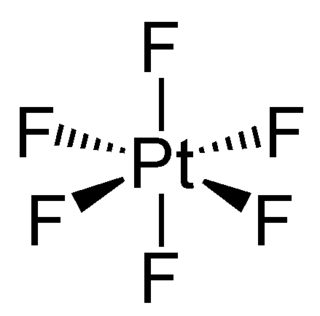
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.
Xenon compounds are compounds containing the element xenon (Xe). After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative atoms fluorine or oxygen. The chemistry of xenon in each oxidation state is analogous to that of the neighboring element iodine in the immediately lower oxidation state.

Xenon oxytetrafluoride is an inorganic chemical compound. It is an unstable colorless liquid with a melting point of −46.2 °C that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica or NaNO
3:

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

A hexafluoroplatinate is a chemical compound which contains the hexafluoroplatinate PtF−6 anion. It is produced by combining substances with platinum hexafluoride.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.

Ruthenium(IV) fluoride is a binary inorganic compound of ruthenium and fluorine with the formula RuF4.
Nickel tetrafluoride is an inorganic compound with a chemical formula NiF4.














