 | |
| Names | |
|---|---|
| IUPAC name tetraiodoplatinum | |
| Other names Platinum tetraiodide, platinic iodide, platinum(4+) tetraiodide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.280 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| I4Pt | |
| Molar mass | 702.702 g·mol−1 |
| Appearance | brown crystals |
| Density | 6.06 g/cm3 |
| Melting point | 130 °C (266 °F; 403 K) |
| decomposes in water | |
| Related compounds | |
Related compounds | Iridium tetraiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
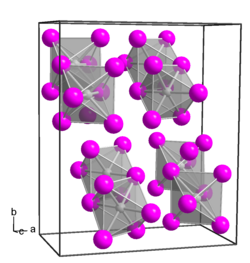
Platinum(IV) iodide is an inorganic compound with the formula PtI4. [1] it is a dark brown diamagnetic solid and is one of several binary iodides of platinum.