
Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.
Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point.

In chemistry, triiodide usually refers to the triiodide ion, I−
3. This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+[I3]−) and ammonium triiodide ([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.
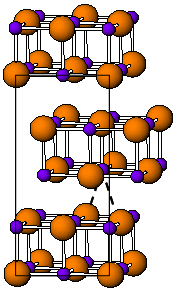
Thallium(I) iodide is a chemical compound with the formula TlI. It is exists as both a solid and high temperature red polymorph. Thallium(I) iodide is one of several water-insoluble metal iodides, along with AgI, CuI, SnI2, SnI4, PbI2, and HgI2.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.
The thallium halides include monohalides, where thallium has oxidation state +1, trihalides in which thallium generally has oxidation state +3, and some intermediate halides containing thallium with mixed +1 and +3 oxidation states. These salts find use in specialized optical settings, such as focusing elements in research spectrophotometers. Compared to the more common zinc selenide-based optics, materials such as thallium bromoiodide enable transmission at longer wavelengths. In the infrared, this allows for measurements as low as 350 cm−1 (28 μm), whereas zinc selenide is opaque by 21.5 μm, and ZnSe optics are generally only usable to 650 cm−1 (15 μm).

Molybdenum(III) iodide is the inorganic compound with the formula MoI3.
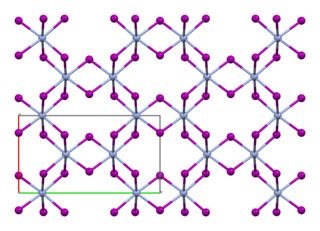
Chromium(III) iodide, also known as chromium triiodide, is an inorganic compound with the formula CrI3. It is a black solid that is used to prepare other chromium iodides.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.
Samarium(III) iodide is an inorganic compound, a salt of samarium and hydroiodic acid with the chemical formula SmI
3.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula Hf I3. It is a black solid.














