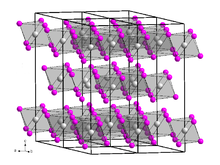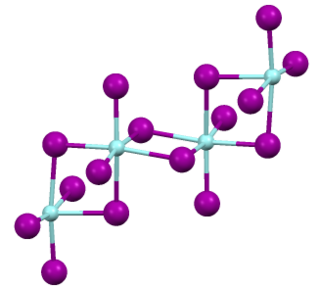
Zirconium(IV) iodide is the chemical compound with the formula ZrI4. It is the most readily available iodide of zirconium. It is an orange-coloured solid that degrades in the presence of water. The compound was once prominent as an intermediate in the purification of zirconium metal.

Thallium(I) iodide is a chemical compound with the formula TlI. It is exists as both a solid and high temperature red polymorph. Thallium(I) iodide is one of several water-insoluble metal iodides, along with AgI, CuI, SnI2, SnI4, PbI2, and HgI2.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.

Molybdenum(III) iodide is the inorganic compound with the formula MoI3.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3 as well as at least one hydrate.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Thulium(III) iodide is an iodide of thulium, with the chemical formula of TmI3. Thulium(III) iodide is used as a component of metal halide lamps.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.

Ytterbium(III) iodide is one of ytterbium's iodides, with the chemical formula of YbI3.
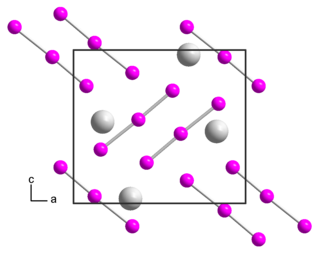
Rubidium triiodide is an inorganic compound with the chemical formula RbI3. It is composed of Rb+ and I−
3.

Dysprosium(II) iodide is an iodide of dysprosium with the chemical formula DyI2.