
Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.

Thorium(IV) nitrate is a chemical compound with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Yttrium oxyfluoride is an inorganic chemical compound with the formula YOF. Under normal conditions, the compound is a colorless solid.

Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C
2BeO
4. It forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is used to prepare ultra-pure beryllium oxide by thermal decomposition.
Praseodymium oxalate is an inorganic compound, a salt of praseodymium metal and oxalic acid with the chemical formula C6O12Pr2. The compound forms light green crystals, insoluble in water, also forms crystalline hydrates.
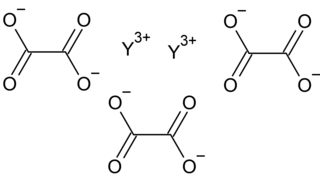
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

Tin(II) oxalate is an inorganic compound, a salt of tin and oxalic acid with the chemical formula SnC
2O
4. The compound looks like colorless crystals, does not dissolve in water, and forms crystalline hydrates.
Neptunium (IV) oxalate is an inorganic compound, a salt of neptunium and oxalic acid with the chemical formula Np(C2O4)2. The compound is slightly soluble in water, forms crystalline hydrates—green crystals.
Samarium(III) oxalate is an inorganic compound, a salt of samarium and oxalic acid with the formula Sm2(C2O4)3. The compound does not dissolve in water, forms a crystalline hydrate with yellow crystals.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.
Actinium (III) nitrate is an inorganic compound, actinium salt of nitric acid with the chemical formula Ac(NO3)3. The compound looks like white substance, readily soluble in water.

Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.
Americium(III) nitrate is an inorganic compound, a salt of americium and nitric acid with the chemical formula Am(NO3)3. The compound is soluble in water and radioactive.

Ytterbium (III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.
Erbium(III) nitrate is an inorganic compound, a salt of erbium and nitric acid with the chemical formula Er(NO3)3. The compound forms pink crystals, readily soluble in water, also forms crystalline hydrates.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Promethium(III) nitrate is an inorganic compound, a salt of promethium and nitric acid with the chemical formula Pm(NO3)3. The compound is radioactive, soluble in water and forms crystalline hydrates.
Polonium tetranitrate is an inorganic compound, a salt of polonium and nitric acid with the chemical formula Po(NO3)4. The compound is radioactive, forms white crystals.














