 Hexahydrate | |
 | |
| Names | |
|---|---|
| IUPAC name Nickel(II) nitrate | |
| Other names Nickel nitrate Nickelous nitrate Nitric acid, nickel(2+) salt | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.774 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 2725 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Ni(NO3)2 | |
| Molar mass | 182.703 g/mol (anhydrous) 290.79 g/mol (hexahydrate) |
| Appearance | emerald green hygroscopic solid |
| Odor | odorless |
| Density | 2.05 g/cm3 (hexahydrate) |
| Melting point | 56.7 °C (134.1 °F; 329.8 K) (hexahydrate) |
| Boiling point | 120–145 °C (248–293 °F; 393–418 K) (hexahydrate, decomposes to basic nickel nitrate) [1] |
| 243 (hexahydrate) g/100ml (0 °C) [2] | |
| Solubility | soluble in ethanol |
| +4300.0·10−6 cm3/mol (+6 H2O) | |
Refractive index (nD) | 1.422 (hexahydrate) |
| Structure | |
| monoclinic (hexahydrate) | |
| Hazards | |
| GHS labelling: | |
     | |
| Danger | |
| H272, H302, H315, H317, H318, H332, H334, H341, H350, H360, H372, H410 | |
| P201, P202, P210, P220, P221, P260, P261, P264, P270, P271, P272, P273, P280, P281, P285, P301+P312, P302+P352, P304+P312, P304+P340, P304+P341, P305+P351+P338, P308+P313, P310, P312, P314, P321, P330, P332+P313, P333+P313, P342+P311, P362, P363, P370+P378, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 1620 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions | Nickel(II) sulfate Nickel(II) chloride |
Other cations | Palladium(II) nitrate |
Related compounds | Cobalt(II) nitrate Copper(II) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
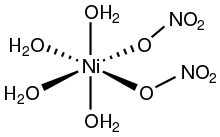
Nickel (II) nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates have also been reported: Ni(NO3)2.9H2O, Ni(NO3)2.4H2O, and Ni(NO3)2.2H2O. [3]
Contents
It is prepared by the reaction of nickel oxide with nitric acid:
- NiO + 2 HNO3 + 5 H2O → Ni(NO3)2.6H2O
The anhydrous nickel nitrate is typically not prepared by heating the hydrates. Rather it is generated by the reaction of hydrates with dinitrogen pentoxide or of nickel carbonyl with dinitrogen tetroxide: [3]
- Ni(CO)4 + 2 N2O4 → Ni(NO3)2 + 2 NO + 4 CO
The hydrated nitrate is often used as a precursor to supported nickel catalysts. [3]
