Related Research Articles

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without cleaving bonds. Usually hydrogenolysis is conducted catalytically using hydrogen gas.

Raney nickel, also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney is a registered trademark of W. R. Grace and Company. Other major producers are Evonik and Johnson Matthey.
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum(IV) oxide hydrate, PtO2•H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions.

Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.
Platinum on carbon, often referred to as Pt/C, is a form of platinum used as a catalyst. The metal is supported on activated carbon in order to maximize its surface area and activity.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
Palladium black is a coarse, sponge-like form of elemental palladium which offers a large surface area for catalytic activity. It is used in organic synthesis as a catalyst for hydrogenation reactions.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.

2,5-Bis(hydroxymethyl)furan (BHMF) is a heterocyclic organic compound, and is a derivative of a broader class of compounds known as furans. It is produced from cellulose and has received attention as a biofeedstock. It is a white solid, although commercial samples can appear yellowish or tan.
Nickel boride is the common name of materials composed chiefly of the elements nickel and boron that are widely used as catalysts in organic chemistry. Their approximate chemical composition is Ni2.5B, and they are often incorrectly denoted "Ni
2B" in organic chemistry publications.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.
Cobalt borides are inorganic compounds with the general formula CoxBy. The two main cobalt borides are CoB and Co2B. These are refractory materials.
Rhodium-platinum oxide , or Nishimura's catalyst, is an inorganic compound used as a hydrogenation catalyst.
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.
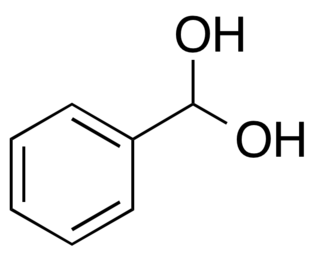
Phenylmethanediol is an organic compound that is a geminal diol, the hydrate of benzaldehyde. It is a short-lived intermediate in some chemical reactions, such as oxidations of toluene and benzaldehyde and the reduction of benzoic acid.

Teruaki Mukaiyama was a Japanese organic chemist. One of the most prolific chemists of the 20th century in the field of organic synthesis, Mukaiyama helped establish the field of organic chemistry in Japan after World War II.
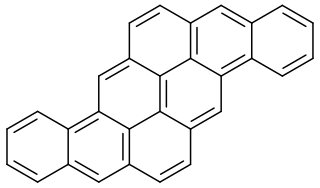
Pyranthrene is a molecule with the chemical formula C30H16.
References
- 1 2 Urushibara, Yoshiyuki; Nishimura, Shigeo (1954). "Procedure for the Preparation of the New Nickel Catalyst". Bulletin of the Chemical Society of Japan. 27 (7): 480. doi: 10.1246/bcsj.27.480 .
- ↑ Sarko, Christopher R.; Dimare, Marcello (2001). "Urushibara Nickel". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ru003. ISBN 0-471-93623-5.
- 1 2 3 Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 19, 36, 94, 123, 166, 204–205. ISBN 9780471396987.
- ↑ Urushibara, Yoshiyuki; Nishimura, Shigeo; Uehara, Hideo (1955). "A New Preparation of Catalytic Nickel". Bulletin of the Chemical Society of Japan. 28 (6): 446. doi: 10.1246/bcsj.28.446 .
- ↑ Taira, Shinichi (1961). "Reduction of Organic Compounds with Urushibara Catalysts under High Pressure. VII. Feature of Various Urushibara Catalysts as Revealed in the Reduction of Benzophenone". Bulletin of the Chemical Society of Japan. 34 (2): 261–270. doi: 10.1246/bcsj.34.261 .
- ↑ Taira, Shinichi (1962). "Reduction of Organic Compounds with Urushibara Catalysts under High Pressure. X. Hydrogenation of 2-Butyne-1,4-diol to cis-2-Butene-1,4-diol with Various Urushibara Catalysts". Bulletin of the Chemical Society of Japan. 35 (5): 840–844. doi: 10.1246/bcsj.35.840 .
- ↑ Urushibara, Yoshiyuki (1952). "A New Method of Catalytic Hydrogenation". Bulletin of the Chemical Society of Japan. 25 (4): 280. doi: 10.1246/bcsj.25.280 .
- ↑ Hata, Kazuo (1972). New Hydrogenating Catalysts: Urushibara Catalysts (1st ed.). Oakland, CA: Wiley. ISBN 9780470358900.