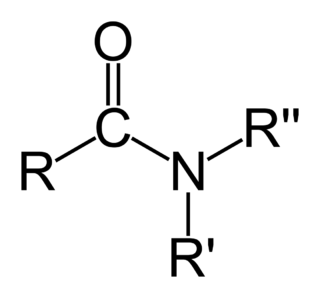
In organic chemistry, an amide ( or or, also known as an organic amide or a carboxamide, is a compound with the general formula RC NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid RC OH with the hydroxyl group –OH replaced by an amine group −NR′R″; or, equivalently, an acyl group RC − joined to an amine group.

A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.

Hydrazones are a class of organic compounds with the structure R
1R
2C=NNH
2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH
2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.

An imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a reducing agent that finds application in chemistry, both in the laboratory and on an industrial scale. It has been tested as pretreatment for pulping of wood, but is too costly to be commercialized. The compound is soluble in alcohols, certain ethers, and water, although it slowly hydrolyzes.

Raney nickel, also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney is a registered trademark of W. R. Grace and Company.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol. modified versions of which were subsequently refined and published in Organic Syntheses.

An iminium cation in organic chemistry is a functional group with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.
Transfer hydrogenation is the addition of hydrogen (H2; dihydrogen in inorganic and organometallic chemistry) to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2. One large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced.

Diethylaluminum cyanide ("Nagata's reagent") is the organoaluminum compound with formula ((C2H5)2AlCN)n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones.
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
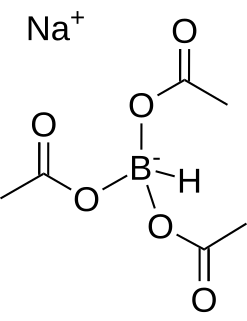
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na(CH3COO)3BH. Like other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid:

Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidines.

Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. A pale yellow liquid, benzophenone imine is used as a reagent in organic synthesis.














